2025
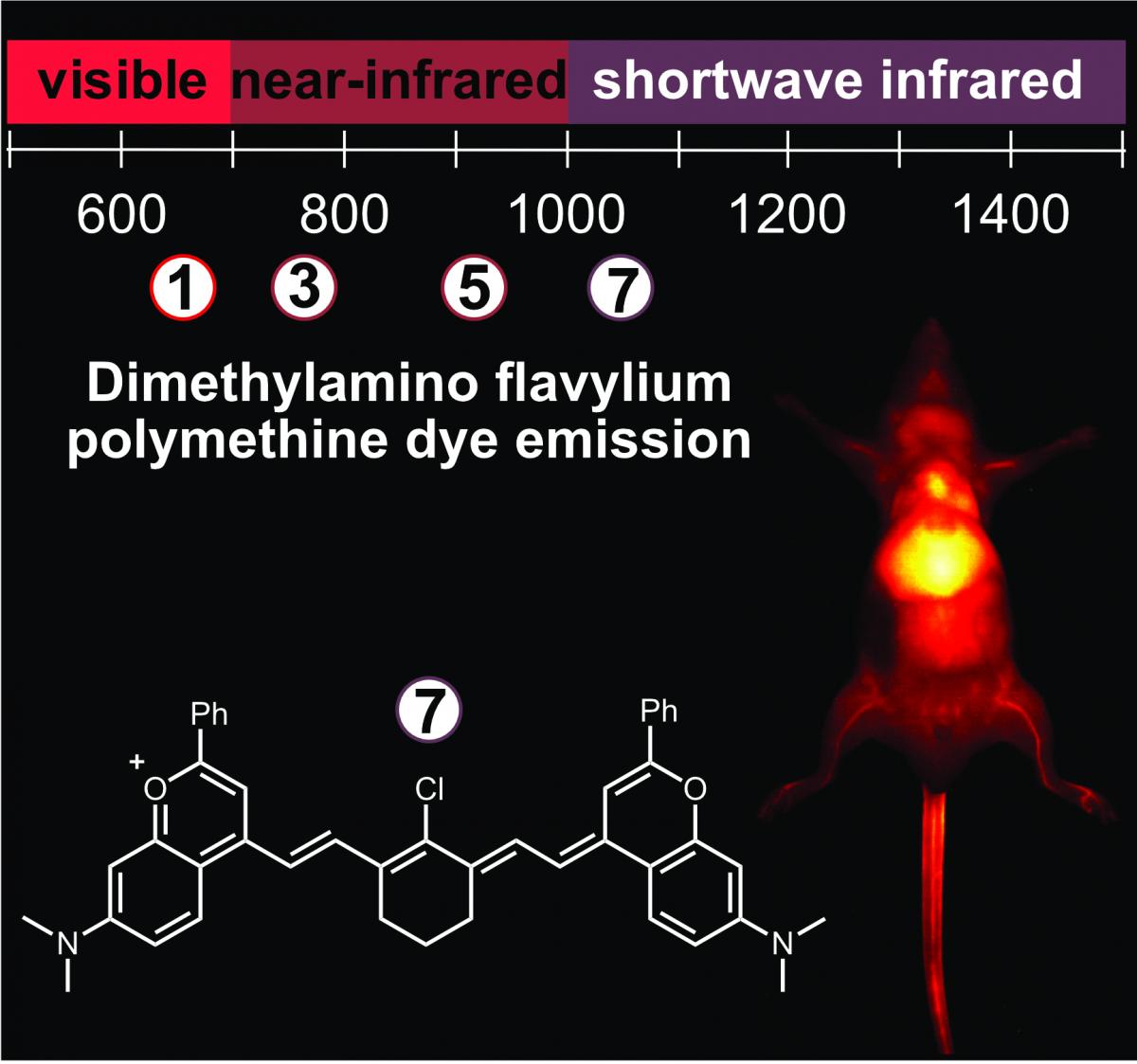
83. Spearman, A.L.; Lin, E.Y.; Mobley, E.B.; Chmyrov, A.; Arús, B.A.; Turner, D.; Garcia, C.; Bui, K.; Rowlands, C.; Bruns, O.T.; Sletten, E.M.* “High-resolution multicolor shortwave infrared dynamic in vivo imaging with chromenylium nonamethine dyes.” J. Am. Chem. Soc. 2025, 147(20), 17384–17393.
First posted here: ChemRxiv. 2024, DOI: 10.26434/chemrxiv-2024-dxdr9.
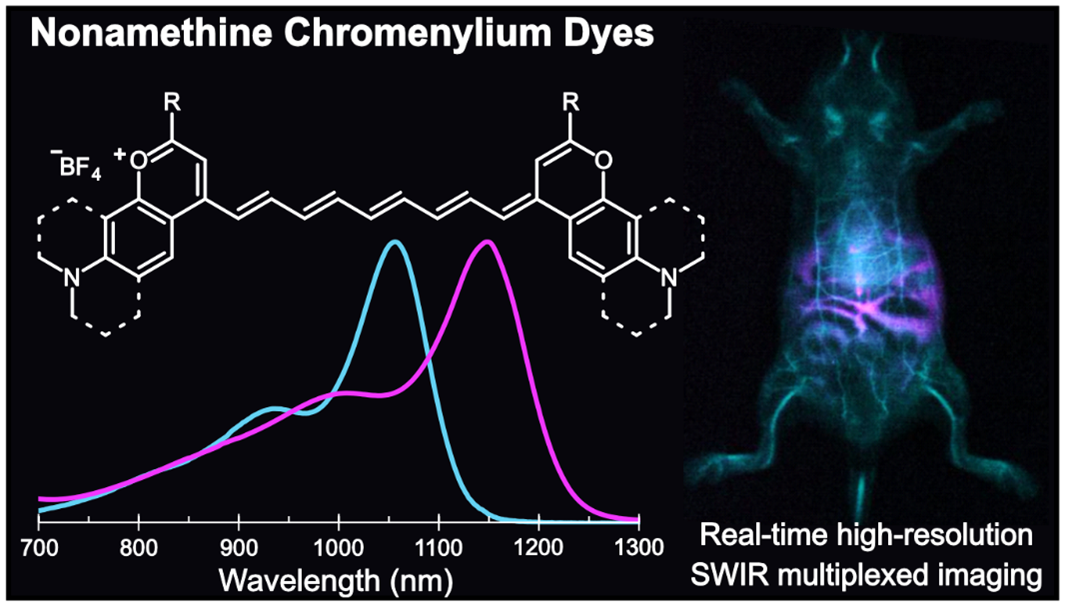
Imaging in the shortwave infrared (SWIR) region offers fast, high-resolution visualization of in vivo targets in a multiplexed manner. These methods require bright, bathochromically shifted fluorescent dyes with sufficient emission at SWIR wavelengths–ideally above 1500 nm for high-resolution deep tissue imaging. Polymethine dyes are a privileged class of contrast agents due to their excellent absorption and high degree of modularity. In this work, we push flavylium and chromenylium dyes further into the SWIR region through polymethine chain extension. This panel of nonamethine dyes boasts absorbances as red as 1149 nm and tail emission beyond 1500 nm. These dyes are the brightest organic fluorophores at their respective bandgaps to date. Using two nonamethine dyes, Chrom9 and JuloFlav9, we performed two-color all-SWIR multiplexed imaging, enhancing the depths and resolutions able to be obtained in multicolor SWIR imaging with small molecule contrast agents. Finally, we combine the nonamenthine dyes with other SWIR-emissive fluorophores and demonstrate five-color awake imaging in an unrestrained mouse, simultaneously pushing the multiplexing, resolution, and speed limits of in vivo optical imaging.
82. Garcia, C.A.; Mobley, E.B.; Lin, E.Y.; Bui, K.; Sletten, E.M.* “Palladium-catalyzed functionalization of shortwave infrared heptamethine fluorophores expands their in vivo utility.” JACS Au. 2025, 5(5), 2089–2101.
Check out the cover art associated with this article!
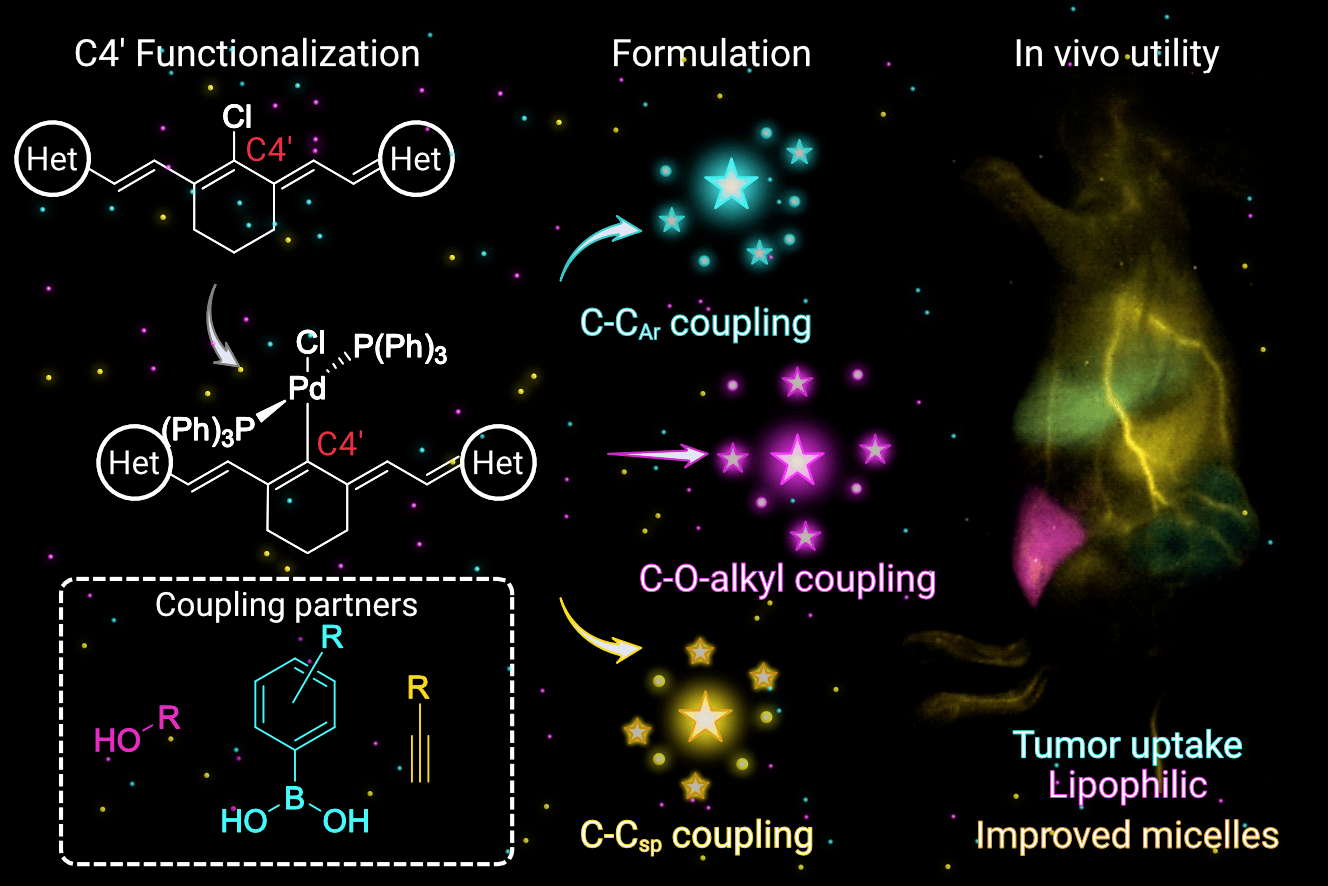
Fluorescence imaging in the near-infrared (NIR, 700–1000 nm) and shortwave infrared (SWIR, 1000–2000 nm) regions is advantageous for studying mammals. This work applies palladium-catalyzed coupling methods to functionalize flavylium and chromenylium SWIR polymethine fluorophores, which are challenging substrates due to their small HOMO–LUMO gaps. These chemistries include Suzuki-Miyaura and Sonogashira couplings as well as an unprecedented coupling of alcohol substrates to ultimately achieve a panel of C–CAr, C–Csp, and C–O-alkyl functionalized SWIR fluorescent heptamethine dyes. The photophysical properties of the resulting fluorophores are analyzed against Hammett parameters to produce predictive metrics for absorption maxima. These metrics are strategically applied in the design of laser-matched, SWIR-emissive, chromenylium heptamethine dyes. Added functionalities advance the utility of SWIR fluorophores by increasing brightness in micelle formulations, modulating lipophilicity for alternative delivery vehicles, and enabling bioconjugation to targeting moieties. Ultimately, three functionalized fluorophores are employed in concert to achieve multicolor excitation-multiplexed imaging in murine cancer models.
81. Ready, A.; Tej Raviprolu, V.*; Kerr, T.; Treacy, J.W.; Matsumoto, M.; Hammer, P.; Sletten, E.M.; Houk, K.N.; Spokoyny, A.* “Carboranes without cage carbons: closo-Dodecaborate mimics of neutral closo-carboranes.” ChemRxiv. 2025, DOI: 10.26434/chemrxiv-2025-w4vvr.
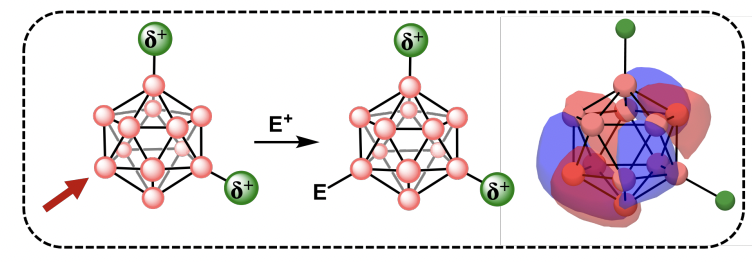
Substituted two-dimensional aromatic systems, such as arenes, exhibit well-established reactivity patterns at specific sites, largely due to the pronounced electronic directing effects of attached substituents. In contrast, the regioselectivity of three-dimensional aromatic molecules as a function of substituents remains less understood and documented. In this work, we demonstrate that a series of closo-dodecaborate ([B12H12]2-) cluster isomers containing two -NMe₃⁺ moieties exhibit unprecedented regioselective reactivity at boron vertices farthest from the charged substituents. Through a combination of theoretical and experimental studies, we reveal that these boron clusters display near-perfect regioselectivity with multiple electrophiles, ultimately enabling vertex differentiation chemistry within these systems. This observed phenomenon closely parallels the reactivity patterns typically associated with icosahedral closo-carboranes, where a carbon-based vertex induces a strong electronic dipole, leading to pronounced vertex-specific reactivity differences at boron sites. Our findings suggest that these modified closo-dodecaborates serve as electronic analogs of closo-carboranes, achieving similar electronic directing effects without the need for cage-based carbon atoms. Instead, exopolyhedral substituents alone govern the regioselective behavior, expanding the potential for tailored functionalization in boron cluster chemistry.
80. Wilson, Q.D.; Lin, H.H.; Lin, E.Y.; Chen, L.; Sletten, E.M.* “Exploiting flavylium merocyanine dyes for intrinsic, multiplexed labeling of the endoplasmic reticulum.” Anal. Chem. 2025, 97(10), 5595–5604.
First posted here: ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-c77hn.
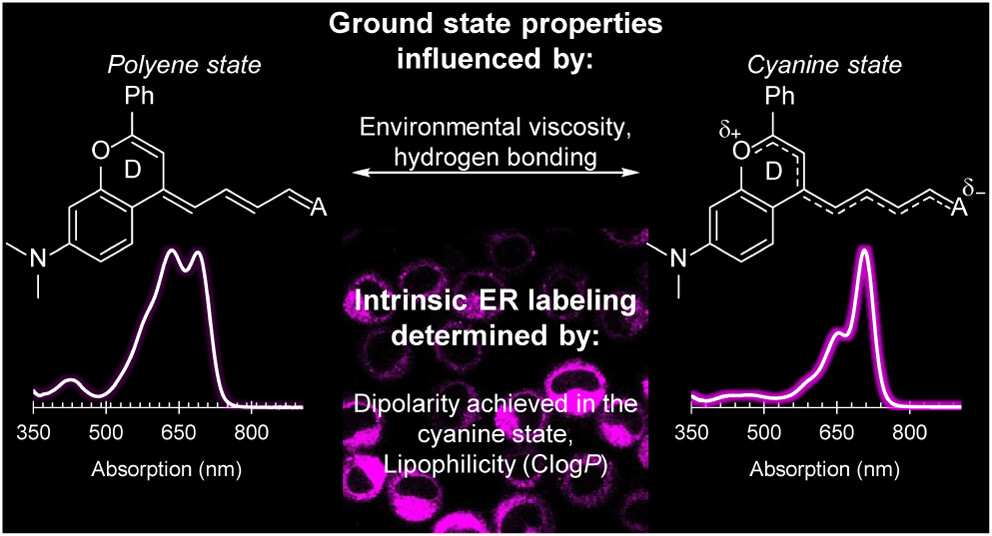
Merocyanine dyes are a versatile class of donor–acceptor polymethine dyes that exhibit unique properties depending on their structural makeup and surrounding environment. Scaffolds that favor the cyanine state (i.e., narrow, red-shifted absorption and high fluorescence quantum yields) in biologically relevant settings are highly advantageous for multiplexed labeling experiments, but remain limited by their visible absorption. Herein, we synthesize a new class of far-red (650–700 nm) to near-infrared (NIR, 700–1000 nm) flavylium merocyanine dyes and demonstrate that they favor the cyanine state with increasing solvent viscosity and hydrogen bond donation. We leverage these advantageous properties for live cell labeling, where we observed intrinsic targeting to the endoplasmic reticulum (ER) and lipid droplets, and minimal crosstalk with commercial stains. We reveal that intrinsic ER labeling is achieved by the dipolarity in the cyanine state and lipophilicity (ClogP) of the merocyanine architecture, making this class of dyes a simple, red-shifted alternative to the more structurally complex ER stains currently available.
79. Garcia, J.A.; Zhu, L.; Vergara Mendez, A.; Sletten, E.M.* “Acid-cleavable poly(oxazoline) surfactants.” Polym. Chem. 2025, 16, 1393–1399.
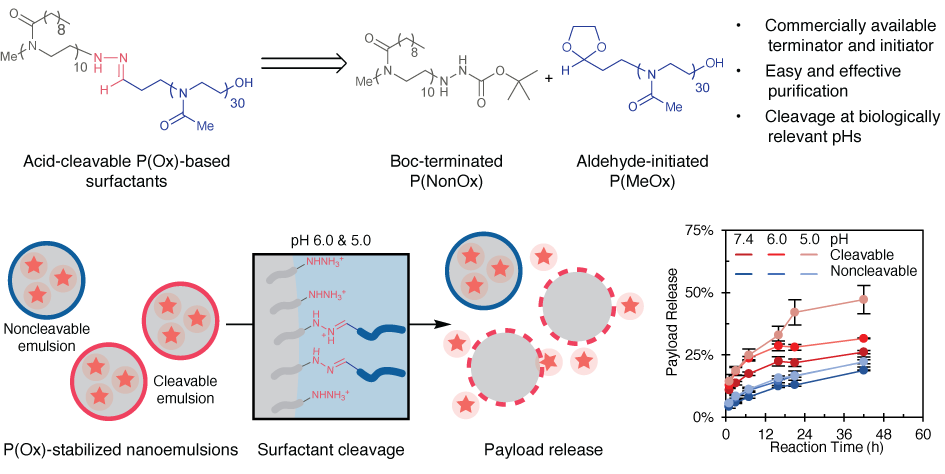
The acidic tumor microenvironment and late endosomes present a promising target for stimuli-responsive nanotherapeutics. Acid-cleavable surfactants, particularly those with hydrazone linkages, offer enhanced stability outside the cell while enabling efficient intracellular payload release. Herein, we report the synthesis of a new hydrazone-linked poly(oxazoline) (POx)-based diblock copolymer surfactant. This surfactant cleaves in a pH-dependent manner going from pH 7.4 down to pH 5.0. We then demonstrate the ability of nanoemulsion encapsulated payloads to partition into cell membrane mimics only after cleavage of the surfactant. Through this system, we were able to increase the amount of payload release from 26% to 47% over 42 hours through pH changes. In all, this work demonstrates a viable route to create POx-based nanomaterials with controlled release capabilities in biologically relevant conditions, and is a promising platform for advancing the endosomal escape and cancer targeting of nanomaterials.
78. Mobley, E.B.; Lin, E.Y.; Sletten, E.M.* “Chromenylium star polymers: Merging water solubility and stealth properties with shortwave infrared emissive fluorophores.” ACS Cent. Sci. 2025, 11(2), 208–218.
Learn more about “SWIR dyes–The eras tour” in Luke Lavis’ feature article on this work: “Stars by the pocketful” in ACS Cent. Sci.
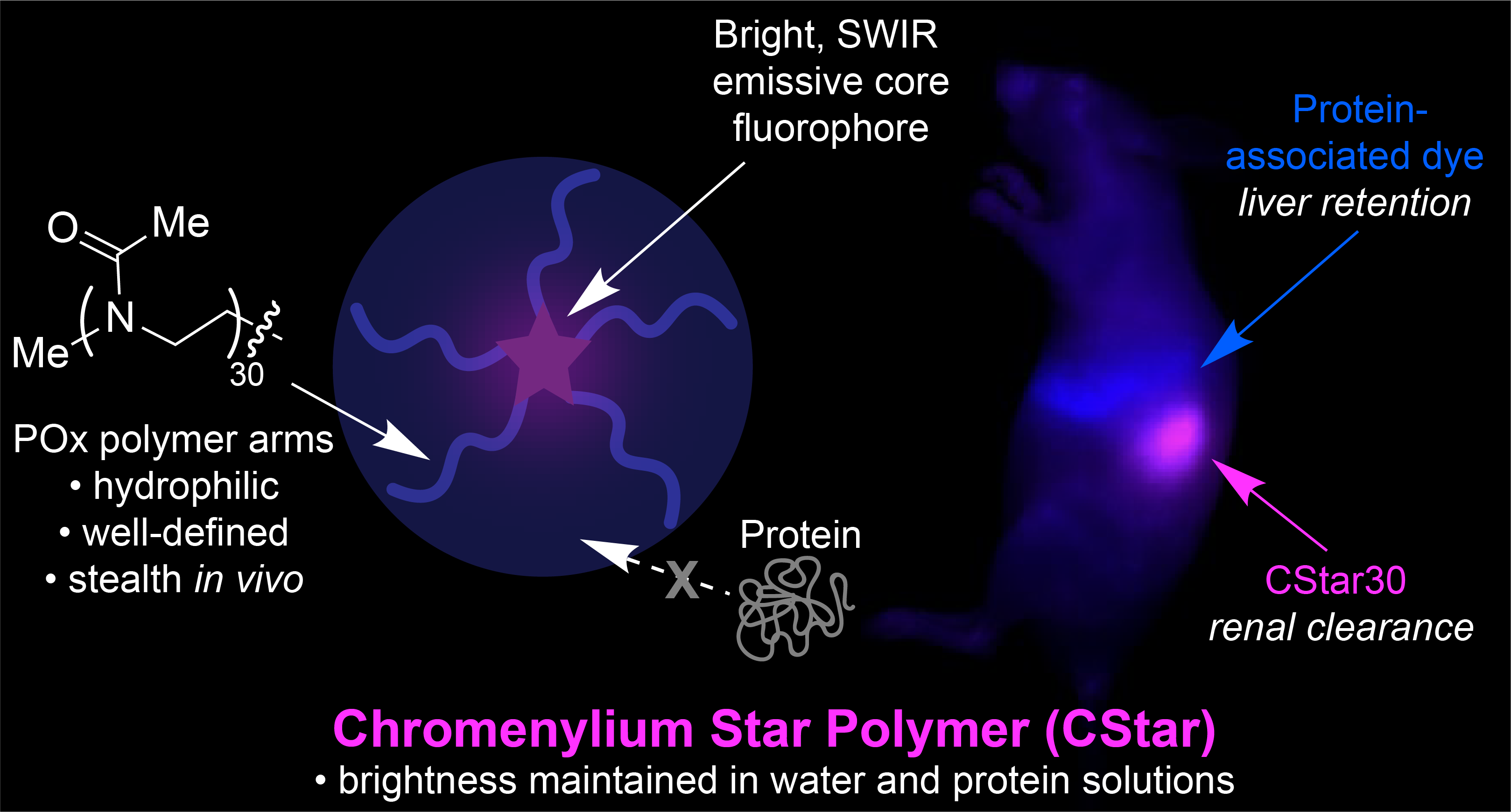
Advancing in vivo imaging utility with these shortwave infrared (SWIR) emissive polymethine dyes is primarily limited by hydrophobicity and/or nonspecific protein association. Herein, we take a distinct approach to combine hydrophilicity and stealth behavior to construct bright, SWIR emissive chromenylium fluorophores by employing a well-defined poly(2-methyl-2-oxazoline) (POx) star polymer architecture, which we refer to as chromenylium stars, or “CStars.” Of these polymer-shielded dyes, the variant containing five POx chains (CStar30) boasts particularly enhanced aqueous solubility and SWIR brightness, enabling high-resolution SWIR imaging in mice. The swift renal clearance and stealth behavior displayed in vivo also achieves improved noninvasive visualization of the lymphatic system. Further, CStar’s orthogonal biodistribution to an FDA-approved dye, indocyanine green (ICG), facilitates excitation-multiplexed SWIR imaging in two colors to achieve simultaneous visualization of both fluid dynamics and protein dynamics in the same animal in real time at video-rate frame counts.
77. Hartung, K.M.; Sletten, E.M.* “Achieving olympicene functionalization three ways.” J. Org. Chem. 2025, 90(1), 889–893.
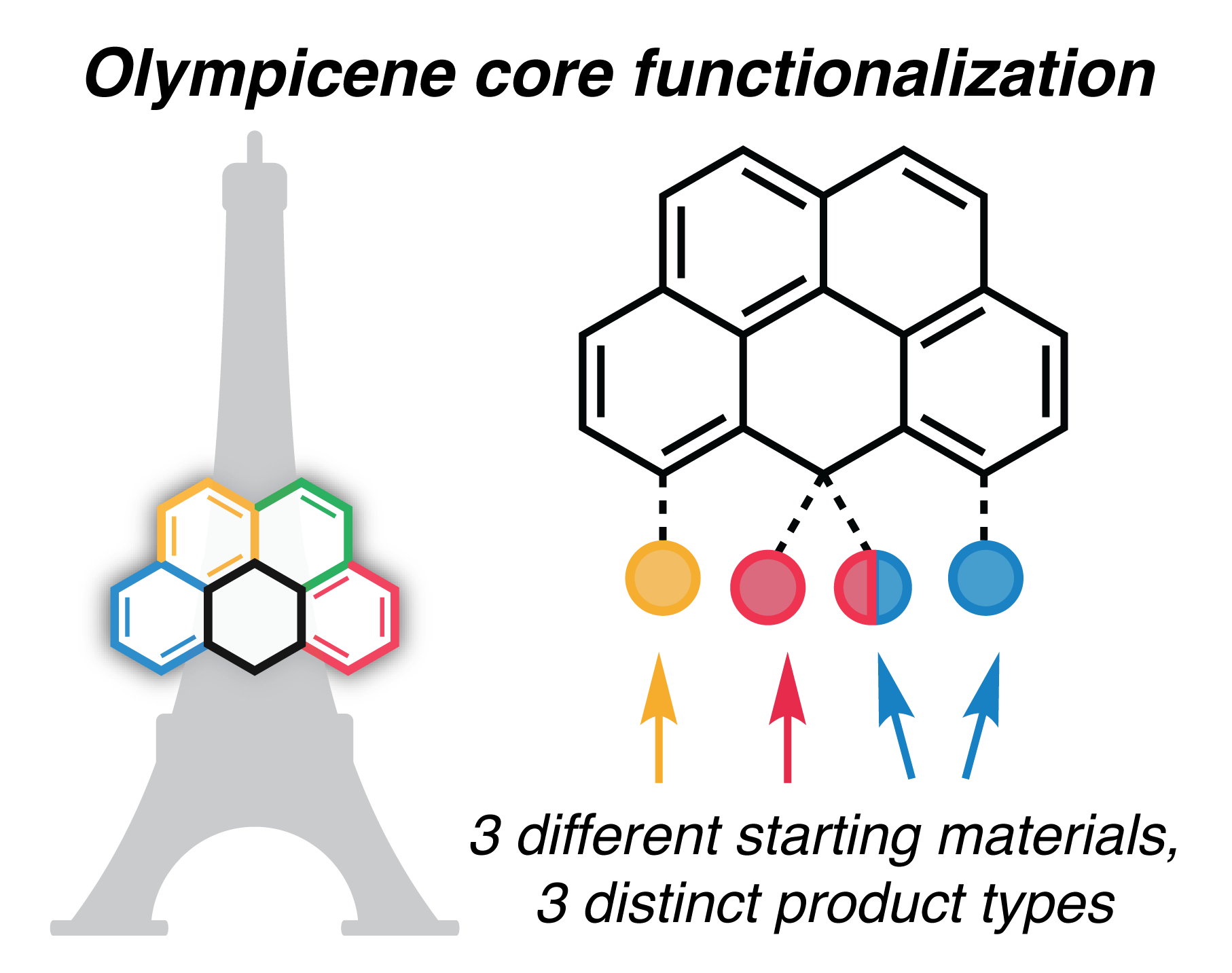
Polycyclic aromatic hydrocarbons (PAHs) and their derivatives are of interest in many fields, from materials science to supramolecular chemistry. 6H-Benzo[cd]pyrene, or olympicene, is a PAH bearing a central sp3-carbon bridge. We sought methods to modify this center position while maintaining stable tetrahedral geometry. Here, we characterize reactivity patterns of three olympicene core starting materials, with two of the three leading to center functionalization of the olympicene core, including the first use of 5H-benzo[cd]pyren-5-one as an olympicene synthon.
2024
76. van de Wouw, H.L.; Yen, S.; Valet, M.; Garcia, J.A.; Gomez, C.O.; Vian, A.; Liu, Y.; Pollock, J.; Pospíšil, P.; Campàs, O.*; Sletten, E.M.* “Non-ionic fluorosurfactants for droplet-based in vivo applications.” Angew. Chem. Int. Ed. 2024, 63(52), e202404956.
First posted here: ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-fbpss.

Fluorocarbon oils are uniquely suited for many biomedical applications due to their inert, bioorthogonal properties. In order to interface fluorocarbon oils with biological systems, nonionic fluorosurfactants are necessary. However, there is a paucity of non-ionic fluorosurfactants with low interfacial tension to stabilize fluorocarbon phases in aqueous environments. We developed non-ionic fluorosurfactants composed of a polyethylene glycol (PEG) segment covalently bonded to a flexible perfluoropolyether (PFPE) segment that confer lower interfacial tensions (IFTs) between a fluorocarbon oil and water. The majority of these custom fluorosurfactants display poor solubility in water, allowing their cointroduction with fluorocarbon oils and minimal leaching. We applied the PEG5PFPE1 surfactant for mechanical force measurements in zebrafish, enabling exceptional sensitivity.
75. Lin, H.H.; Lim, I.; Sletten, E.M.* “Counterion exchange enhances the brightness and photostability of a fluorous cyanine dye.” Chem. Eur. J. 2024, 30(64), e202402514.
First posted here: ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-3rvk6.
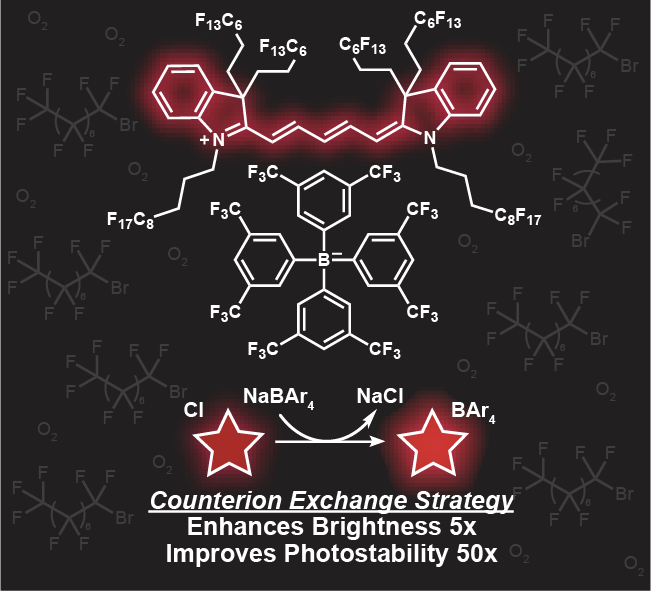
Fluorofluorophores are a unique class of fluorophores that can be solubilized in perfluorocarbons (PFCs) and used to study biological systems. However, because of the low dielectric constant and high oxygen solubility of the fluorous phase, the brightness and photostability of the fluorofluorophores are significantly diminished. Here, we leveraged the tight ion pairing in the fluorous phase to improve the photophysical properties of a fluorous-soluble pentamethine dye (FCy5) via counterion exchange. We found that larger, softer aryl borate counterions promote the ideal polymethine state where charge delocalization across the polymethine chain increases the brightness (6-fold) and photostability (55-fold) of FCy5.
74. Garcia, J.A.; Vergara Mendez, A.; Sletten, E.M.* “Biotin-initiated poly(oxazoline)s.” Macromolecules. 2024, 57(13), 6354–6361.
First posted here: ChemRxiv 2024, DOI: 10.26434/chemrxiv-2024-v0n1h
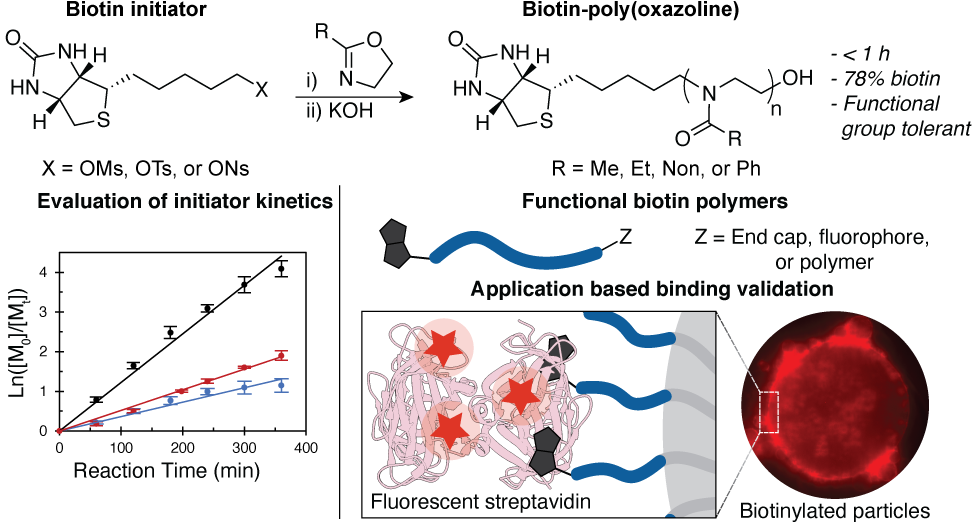
Biotinylated hydrophilic polymers are used in a plethora of chemical and biochemical assays due to the exceptional affinity of biotin for (strept)avidin. Here we report a facile method for the installation of biotin at the end of poly(oxazoline)s (P(Ox)) using an electrophilic biotin initiator. While this method is applicable to many different oxazoline monomers, we focus on biotin-poly(2-methyl-2-oxazoline) as it is a desirable alternative to poly(ethylene glycol)(PEG), which has rising immunogenicity concerns. A further advantage of POx over PEG is the high degree of chemical flexibility that can be imparted, which we showcase through the preparation of varied molecular weight, amphiphilic, and bifunctional POxs. Furthermore, we demonstrate the high efficacy of these biotin polymers through a series of common biochemical assays that frequently utilize the biotin- streptavidin pair.
73. Meador, W.E.‡; Lin, E.Y.‡; Lim, I.; Friedman, H.C.; Ndaleh, D.; Shaik, A.K.; Hammer, N.I.; Yang, B.; Caram, J.R.; Sletten, E.M.*; Delcamp, J.H.* “Silicon-RosIndolizine fluorophores with shortwave infrared absorption and emission profiles enable in vivo fluorescence imaging.” Nat. Chem. 2024, 16, 970–978.
‡ Denotes authors that contributed equally
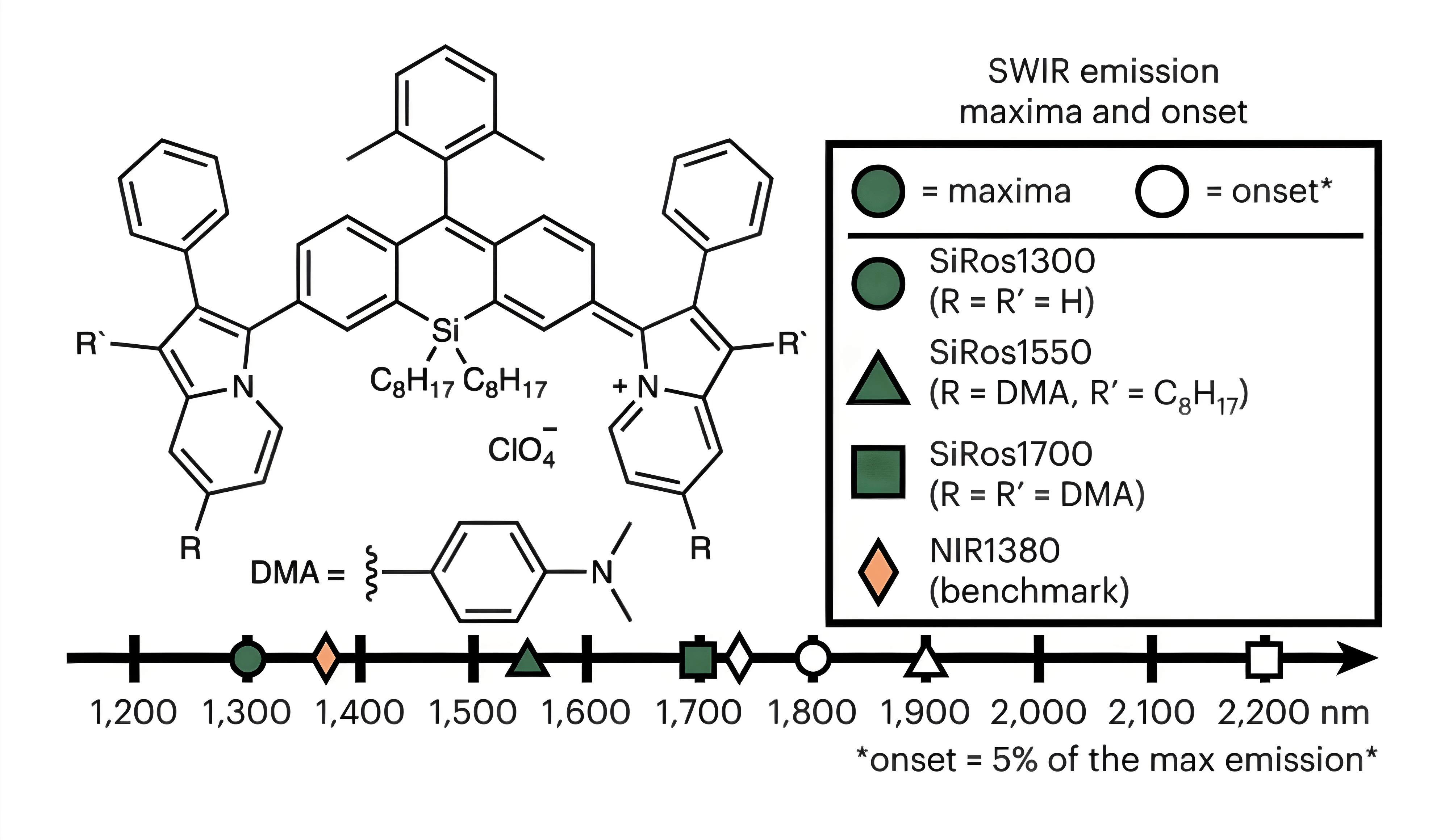
In vivo fluorescence imaging in the shortwave infrared (SWIR, 1,000–1,700 nm) and extended SWIR (ESWIR, 1,700–2,700 nm) regions has tremendous potential for diagnostic imaging. Although image contrast has been shown to improve as longer wavelengths are accessed, the design and synthesis of organic fluorophores that emit in these regions is challenging. Here we synthesize a series of silicon-RosIndolizine (SiRos) fluorophores that exhibit peak emission wavelengths from 1,300–1,700 nm and emission onsets of 1,800–2,200 nm. Using two of the fluorophores (SiRos1300 and SiRos1550), we formulate nanoemulsions and use them for general systemic circulatory SWIR fluorescence imaging of the cardiovascular system in mice. These studies resulted in high-resolution SWIR images with well-defined vasculature visible throughout the entire circulatory system. This SiRos scaffold establishes design principles for generating long-wavelength emitting SWIR and ESWIR fluorophores.
72. Ramos, P.; Friedman, H.; Li, B.Y.; Garcia, C.; Sletten, E.M.; Caram, J.R.; Jang, S.J.* “Non-adiabatic derivative couplings through multiple Franck-Condon modes dictate the energy gap law for near and short-wave infrared dye molecules.” J. Phys. Chem. Lett. 2024, 15(7), 1802–1810.
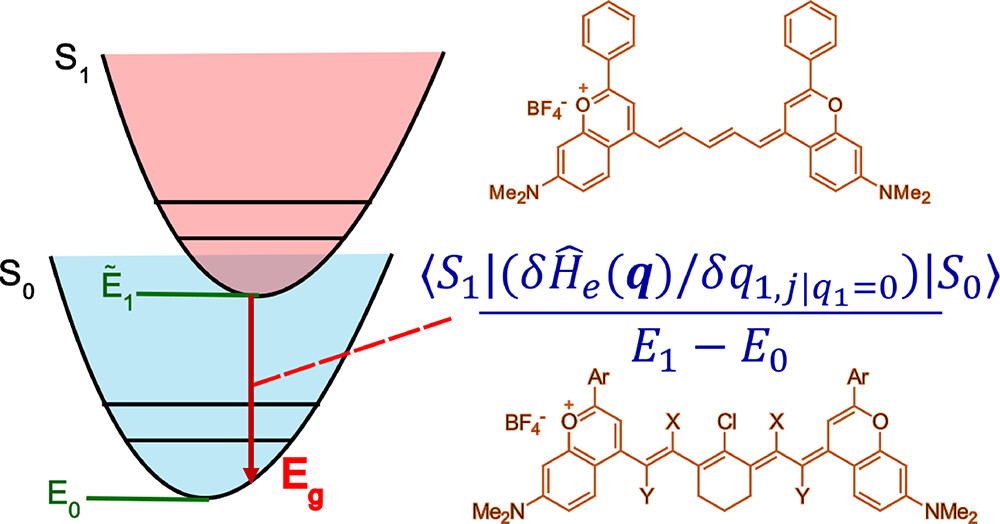
Near infrared (NIR, 700–1000 nm) and short-wave infrared (SWIR, 1000–2000 nm) dye molecules exhibit significant nonradiative decay rates from the first singlet excited state to the ground state. While these trends can be empirically explained by a simple energy gap law, detailed mechanisms of nearly universal behavior have remained unsettled for many cases. Here, it is shown that the first derivative nonadiabatic coupling terms serve as major coupling pathways for nonadiabatic decay processes from the first excited singlet state to the ground state for these NIR and SWIR dye molecules and that vibrational modes other than the highest frequency modes also make significant contributions to the rate.
71. Deshmukh, A.P.; Zheng, W.; Chuang, C.; Bailey, A.D.; Williams, J.A.; Sletten, E.M.; Egelman, E.H.; Caram, J.R.* “Near-atomic-resolution structure of J-aggregated helical light-harvesting nanotubes.” Nat. Chem. 2024, 16, 800–808.
First posted here: ChemRxiv 2022, DOI: 10.26434/chemrxiv-2022-5m8sx.
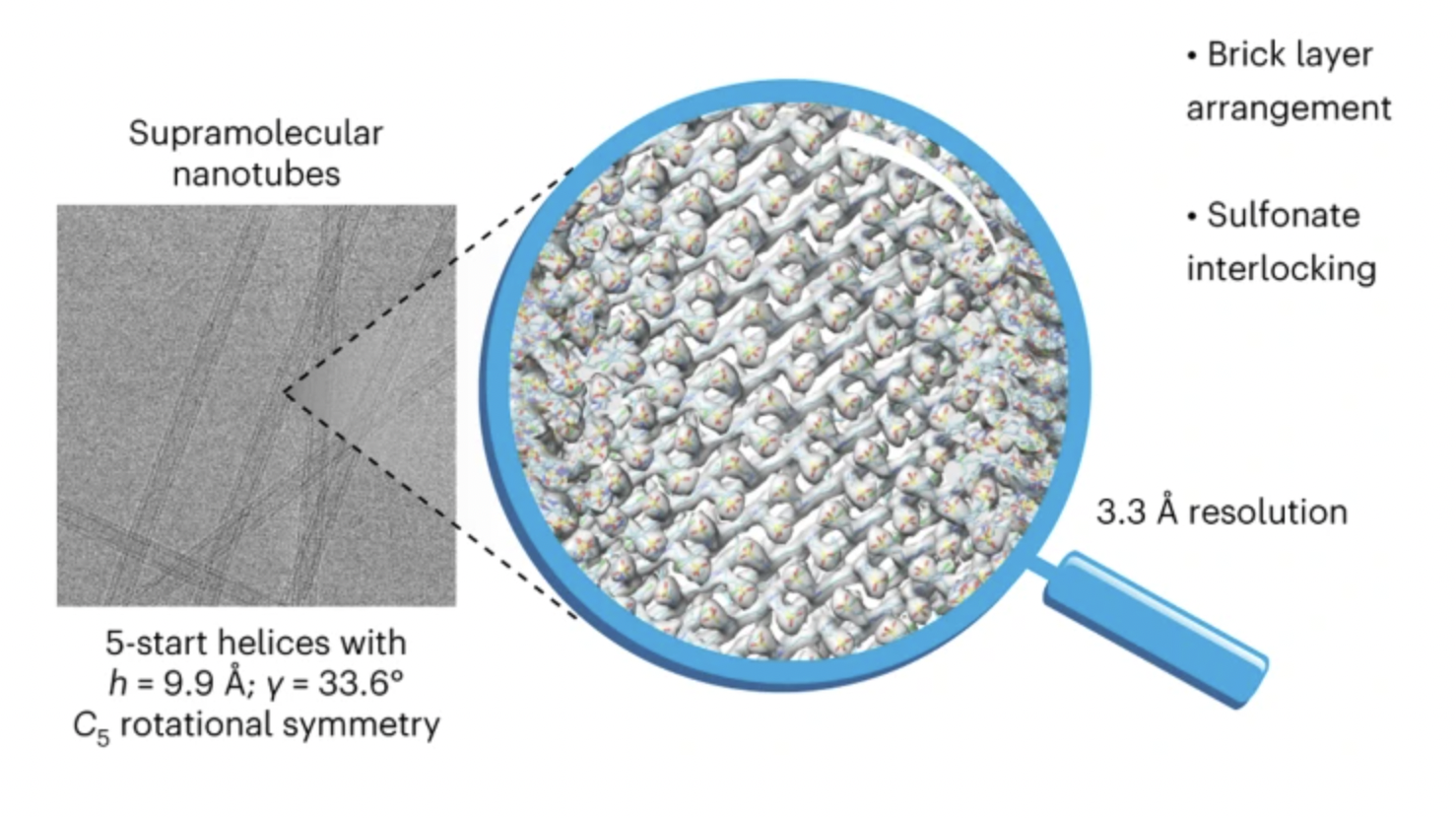
Cryo-electron microscopy has delivered a resolution revolution for biological self-assemblies, yet only a handful of structures have been solved for synthetic supramolecular materials. Here, we present a 3.3 Å structure of prototypical biomimetic light-harvesting nanotubes derived from an amphiphilic cyanine dye (C8S3-Cl). Helical 3D reconstruction directly visualizes the chromophore packing that controls the excitonic properties. Furthermore, we identify a new non-biological supramolecular motif—interlocking sulfonates—that may be responsible for the slip-stacked packing and J-aggregate nature of the light-harvesting nanotubes. This work shows how independently obtained native-state structures complement photophysical measurements and will enable accurate understanding of (excitonic) structure–function properties, informing materials design for light-harvesting chromophore aggregates.
2023
70. Wilson, Q.D.; Sletten, E.M.* “Engineering cyanine cyclizations for new fluorogenic probes.” Nat. Chem. 2023, 16, 3–5.
A News and Views piece for: “A general strategy to develop fluorogenic polymethine dyes for bioimaging.”
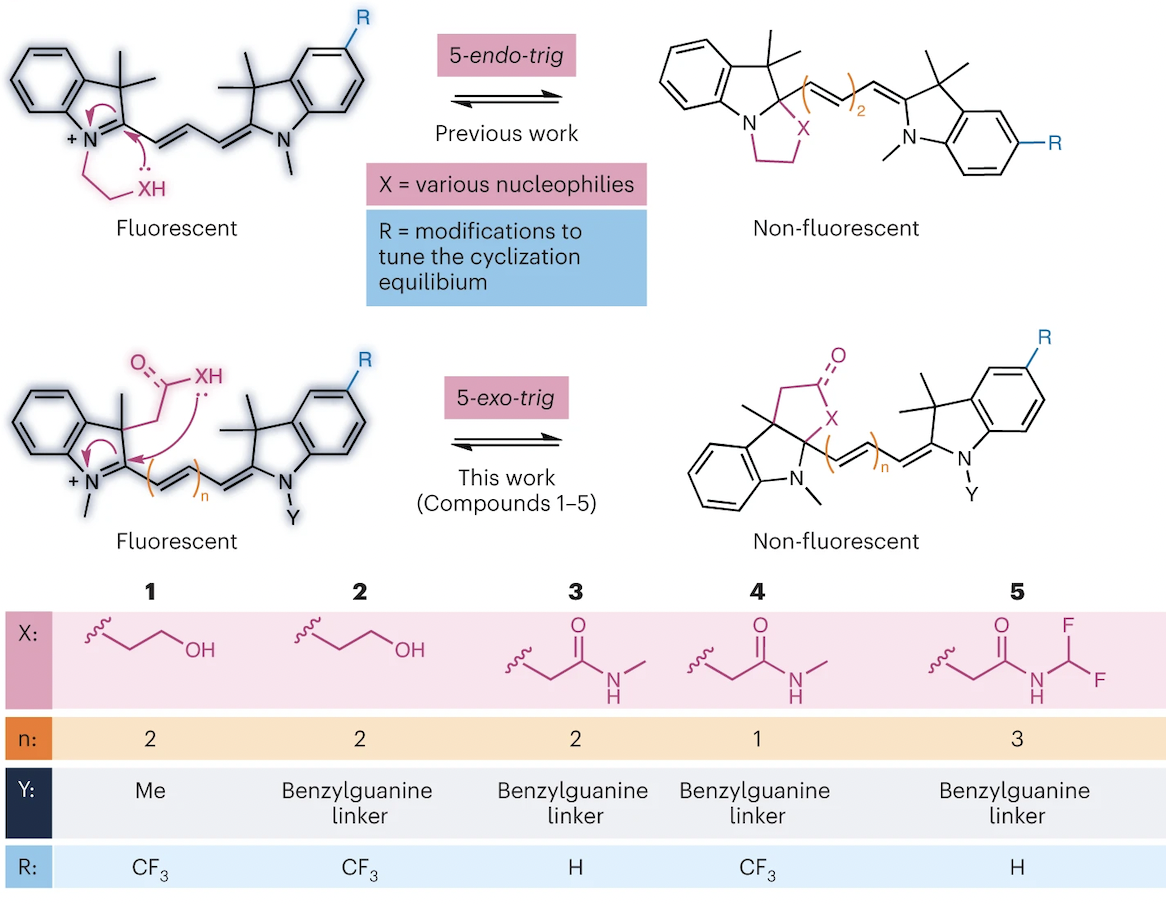
As the need for specific fluorescent probes that enable high sensitivity and super-resolution imaging experiments continues to grow, it is imperative to develop new, well-characterized methods to modulate the emission of fluorophores. Now, a general platform affords visible-to-NIR fluorogenic fluorophores by engineering a simple cyclization event into cyanine dyes.
69. Vian, A.; Pochitaloff, M.; Yen, S.-T.; Kim, S.; Pollock, J.; Liu, Y.; Sletten, E.M.; Campás, O.* “In situ quantification of osmotic pressure within living embryonic tissues.” Nat. Commun. 2023, 14, 7023.
First posted here: bioRxiv 2022, DOI: 10.1101/2022.12.04.519060
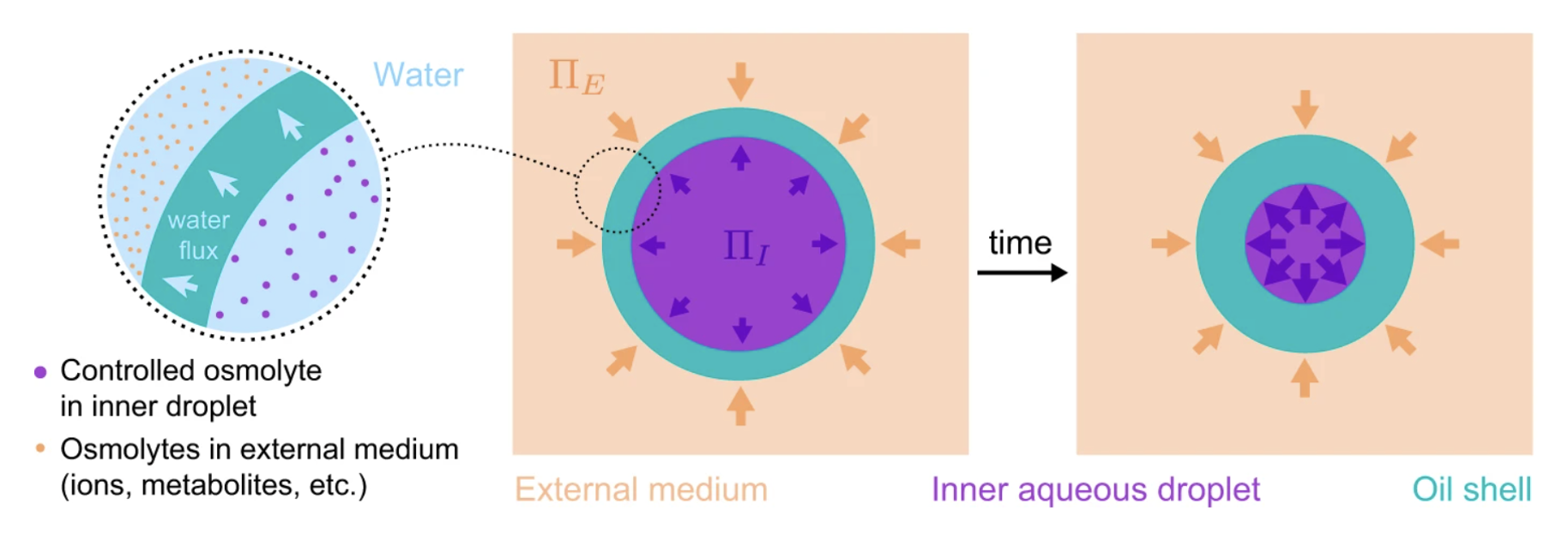
Mechanics is known to play a fundamental role in many cellular and developmental processes. Beyond active forces and material properties, osmotic pressure is believed to control essential cell and tissue characteristics. However, it remains very challenging to perform in situ and in vivo measurements of osmotic pressure. Here we introduce double emulsion droplet sensors that enable local measurements of osmotic pressure intra- and extra-cellularly within 3D multicellular systems, including living tissues. Our results show a balance between intracellular and interstitial osmotic pressures, with values of approximately 0.7 MPa, but a large pressure imbalance between the inside and outside of the embryo. The ability to measure osmotic pressure in 3D multicellular systems, including developing embryos and organoids, will help improve our understanding of its role in fundamental biological processes.
68. Lin, F-C.; van de Wouw, H.L.; Campás, O.; Sletten, E.M.; Zink, J.I.* “Synthesis of fluorous ferrofluids and effects of the nanoparticle coatings on field-and temperature-dependent magnetizations.” Chem Mater. 2023, 35(19), 7957–7966.
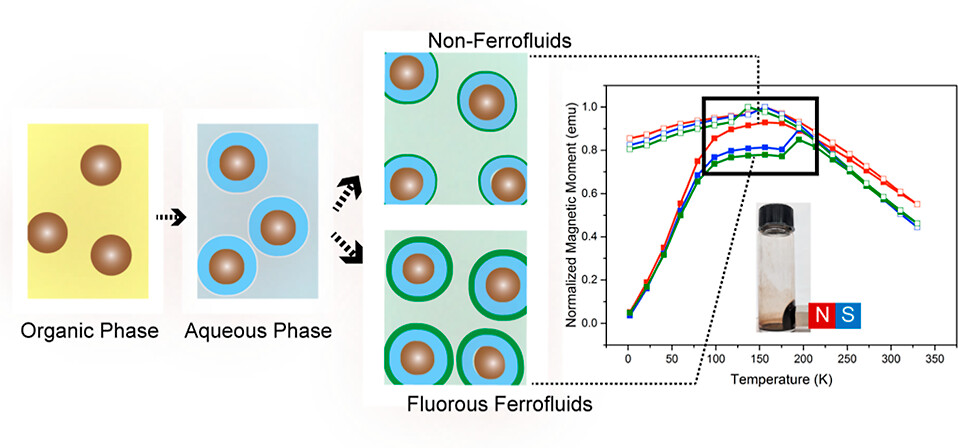
Fluorous ferrofluids are of interest for biomedical applications because of the biorthogonal nature of perfluorocarbons (PFCs). However, the noninteracting nature of PFCs as well as challenges in functionalization of nanoparticle surfaces with fluorous ligands has limited their applications, especially in biomedicine. In this paper, we developed a unique two-phase ligand attachment strategy to render stable fluorous ferrofluids using nonionic surfactants. We explored chemistry–material relationships between different ligands and PFC solvents and found that low-molecular-weight ligands can assist with the installation of high-molecular-weight ligands (4000–8000 g/mol), allowing us to systematically control the size and thickness of ligand functionalization on the nanoparticle surface. The resulting doped MnFe2O4 fluorous ferrofluid has the highest combination of stability and magnetization reported to date.
67. Jia, S.; Lin, E.Y.; Mobley, E.B.; Lim, I. Guo, L.; Kallepu, S.; Low, P.S.; Sletten, E.M.* “Water-soluble chromenylium dyes for shortwave infrared imaging in mice.” Chem. 2023, 9(12), 3648–3665.
First posted here: ChemRxiv 2022, DOI: 10.26434/chemrxiv-2022-h8rjb.
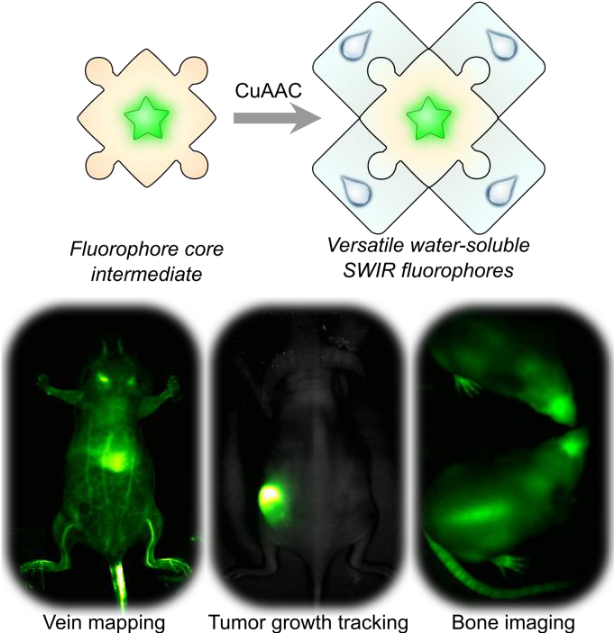
Optical imaging with fluorophores that emit in the low energy wavelengths of light of the shortwave infrared (SWIR, 1000–2000 nm) is advantageous for obtaining high resolution information on living systems, in real-time. However, developing biocompatible fluorophores (i.e., fluorophores that are soluble in water) has been a significant challenge despite recent advances in the field. Here, we report a versatile platform for developing fluorophores that are biocompatible, and functionalized to 1) track tumor cells over long periods of time, 2) reduce the amount of fluorophore required for video-rate imaging in mice models, and 3) target and label bones in awake and moving mice. We showcase these advances with SWIR imaging in mouse models. In the future, we envision that this scaffold will provide valuable insights for facile derivitization of other existing fluorophores to achieve biocompatibility, and ultimately, advances in bioimaging applications.
66. Lingg, J.G.P.; Bischof, T.S.; Arús, B.A.; Cosco, E.D.; Sletten, E.M.; Rowlands, C.J.; Bruns, O.; Chmyrov, A.* “Shortwave-infrared line-scan confocal microscope for deep tissue imaging in intact organs.” Laser Photonics Rev. 2023, 17(11), 2300292.
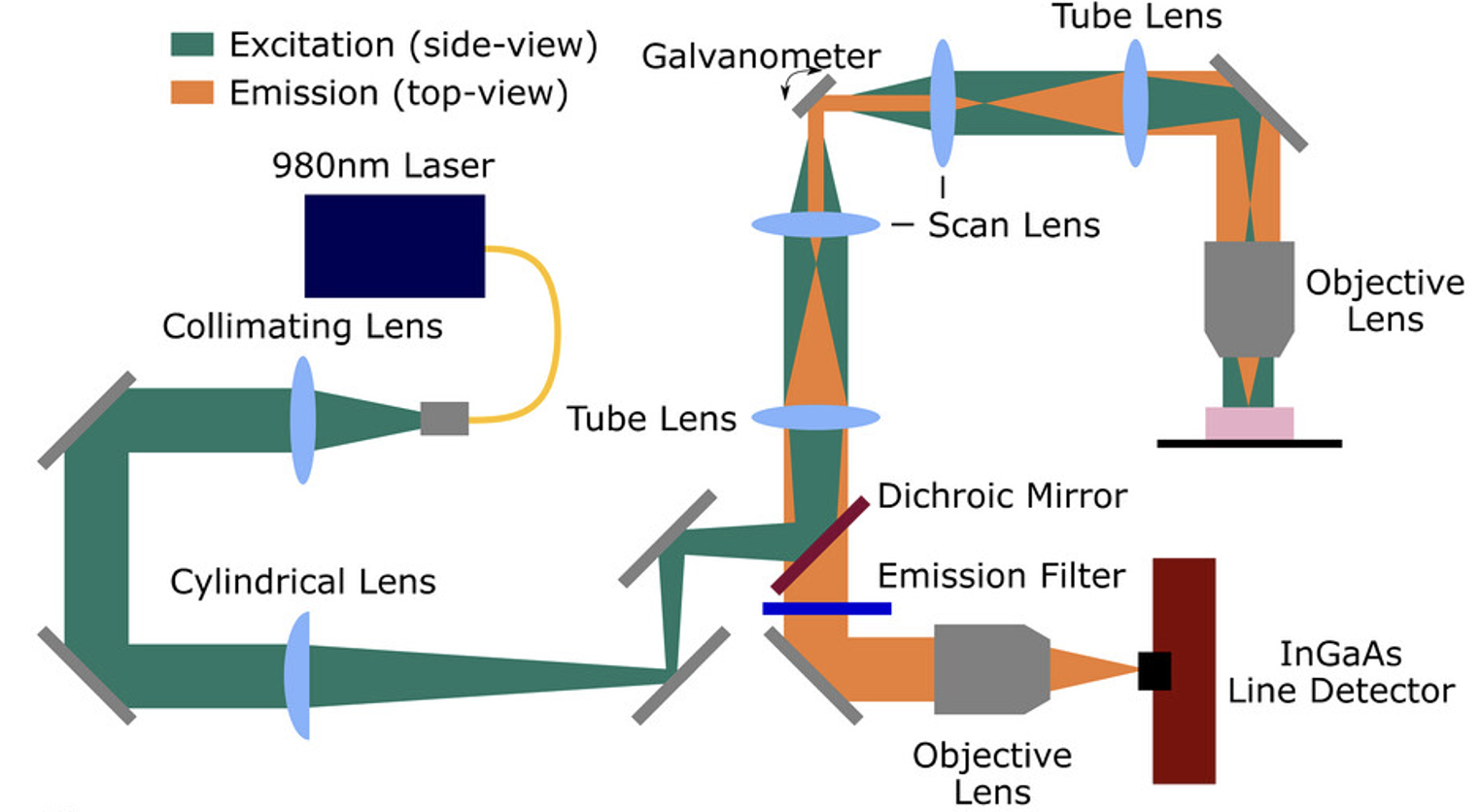
The development of fluorophores with photoemission beyond 1000 nm provides the opportunity to develop novel fluorescence microscopes sensitive to those wavelengths. Imaging at wavelengths beyond the visible spectrum enables imaging depths of hundreds of microns in intact tissue, making this attractive for volumetric imaging applications. Here, a novel shortwave-infrared line-scan confocal microscope is presented that is capable of deep imaging of biological specimens, as demonstrated by visualization of labeled glomeruli in a fixed uncleared kidney at depths beyond 400μm.Imaging of brain vasculature labeled with the near-infrared organic dye indocyanine green, the shortwave-infrared organic dye Chrom7, and rare earth-doped nanoparticles is also shown, thus encompassing the entire spectrum detectable by a typical shortwave-infrared sensitive InGaAs detector.
65. Hartung, K. M.; Sletten, E.M.* “Bioorthogonal chemistry: Bridging chemistry, biology, and medicine.” Chem. 2023, 9(8), 2095–2109.
As chemical biologists sought methods to modify and study biomolecules in their native environments, the need for bioorthogonal chemical reactions emerged. These fast and selective reactions between otherwise inert, abiotic functional groups have enabled exploration of some of the most intriguing and challenging questions in chemical biology. Further, the ability to perform organic reactions in cells and organisms has led to important applications in clinical spaces, and one reaction is now an integral part of a phase 2 trial for treating solid tumors. Given that bioorthogonal chemistry was a recipient of the 2022 Nobel Prize, we expect this field to be even more energized. Here, we highlight some of the most recent studies in this sphere and how these set the stage for where bioorthogonal chemistry is headed.
64. Lim, I.; Lin, E.Y.; Garcia, J.A.; Jia, S.; Sommerhalter, R.E.; Ghosh, S.K.; Gladysz, J.A.; Sletten, E.M.* “Shortwave infrared fluorofluorophores for multicolor in vivo imaging.” Angew. Chem. Int. Ed. 2023, 62(6), e202215200.
First posted here: ChemRxiv 2022, DOI: 10.26434/chemrxiv-2022-vm3jb.
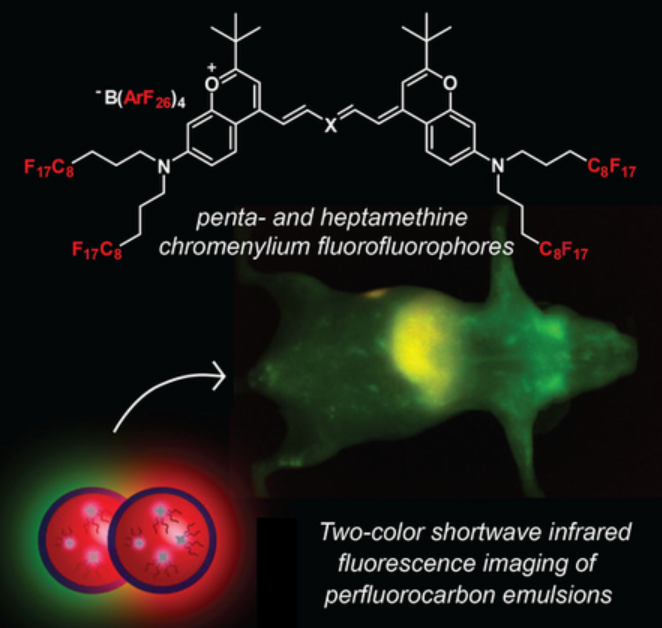
It is critical to develop molecular tools that can help visualize biological processes in living systems. Here we created nanoemulsions housing shortwave infrared (SWIR, 1000–2000 nm) light emitting fluorofluorophores, with fluoro refering to both fluorine and fluorescence. These combine two entities that are safe and minimally interact with any biomolecule: perfluorocarbons and shortwave infrared fluorescence. Two SWIR fluorofluorophores exhibiting the longest emission wavelengths to date were synthesized. Each of these dyes were then placed inside perfluorocarbon nanoemulsions and introduced into mice for simultaneous two-color imaging. Through varying the characteristics of each nanoemulsion, we were able to learn how size and surfactant identity can affect a nanoemulsion’s biodistribution.
63. Arus, B.A.; Cosco, E.D.; Yiu, J.; Balba, I.; Bischof, T.S.; Sletten, E.M.; Bruns, O.T.* “Shortwave infrared fluorescence imaging of peripheral organs in awake and freely moving mice.” Front. Neurosci. 2023, 17, 1135494.
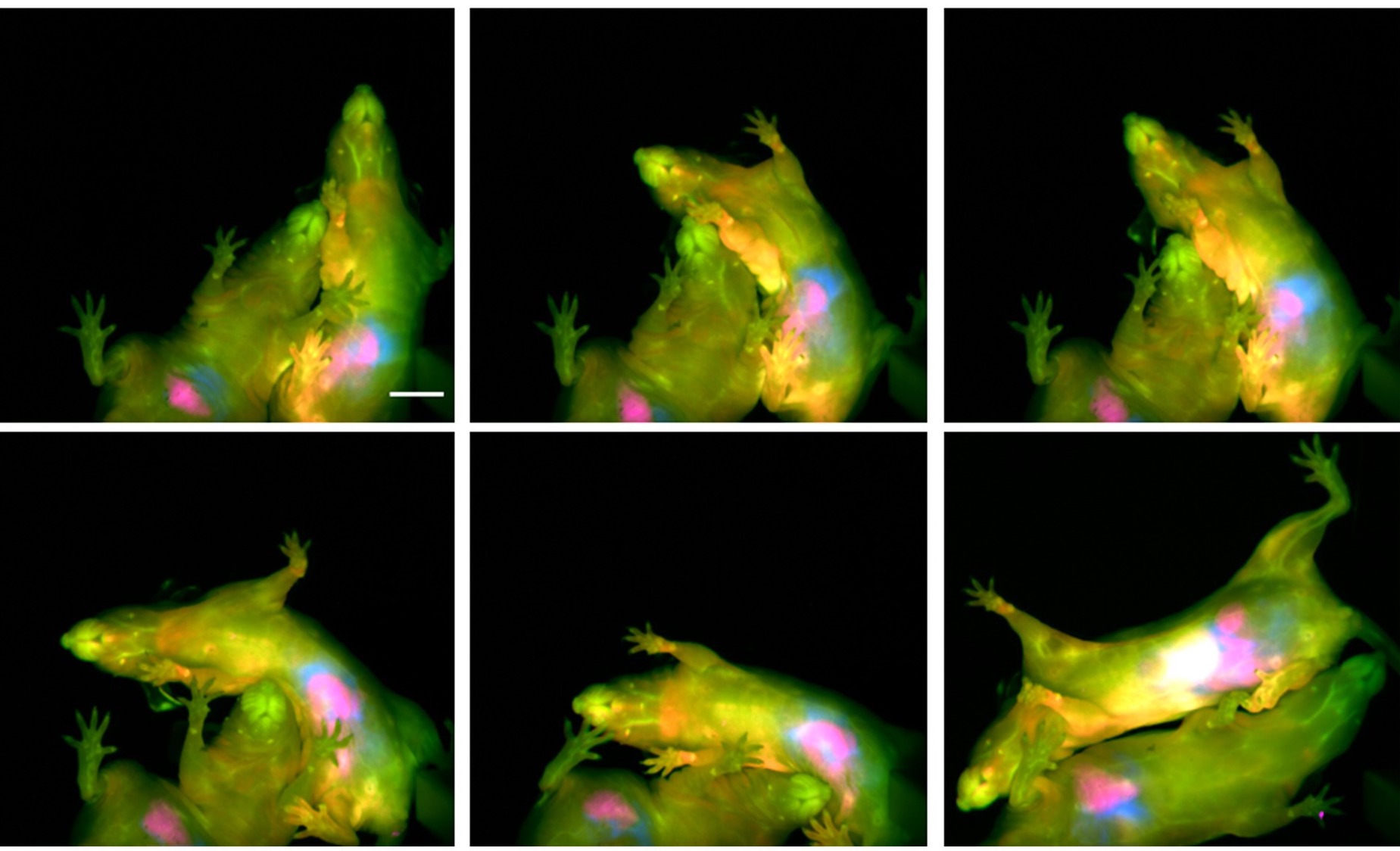
Extracting biological information from awake and unrestrained mice is imperative to in vivo basic and pre-clinical research. Accordingly, imaging methods which preclude invasiveness, anesthesia, and/or physical restraint enable more physiologically relevant biological data extraction by eliminating these extrinsic confounders. In this article, we discuss the recent development of shortwave infrared (SWIR) fluorescent imaging to visualize peripheral organs in freely-behaving mice, as well as propose potential applications of this imaging modality in the neurosciences.
62. Kataki-Anastasakou, A.; Jia, S.; Axtell, J.A.; Sletten, E.M.* “A fluorescent unnatural mannosamine derivative with enhanced emission upon complexation with cucurbit[7]uril.” Isr. J. Chem. 2022, 63(1-2), e202200069.
Dedicated to Carolyn Bertozzi for her inspiration, brilliance, and advocacy; 2022 Wolfe Prize.
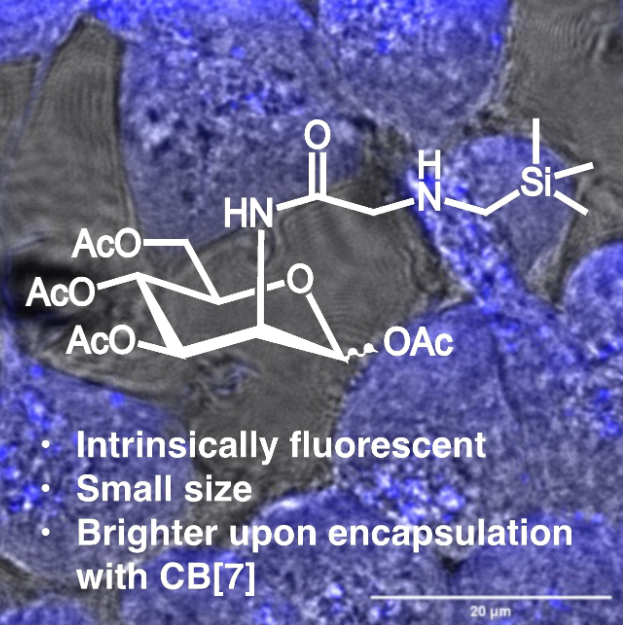
Metabolic incorporation of unnatural functionality on glycans has allowed chemical biologists to observe and affect cellular processes. Recent workhas resulted in glycan-fluorophore structures that allow for direct visualization of glycan-mediated processes, shining light on their role in living systems.This work describes the serendipitous discovery of a small chemical reporter-fluorophore. Investigations into the mechanism of fluorescence arising from(trimethylsilyl)methylglycine appended on mannosamine suggest rigidity and restriction of lone pair geometry contribute to the fluorescent behaviour. In fact, in situ cyclization and encapsulation in cucurbit[7]uril enhance fluorescence to levels that can be observed in live cells.
61. Sica, A.; Hua, A.; Lin, H.H.; Sletten, E.M.; Atallah, T.;* Caram, J.R.* “Spectrally-selective time-resolved emission through Fourier-filtering (STEF).” J. Phys. Chem. Lett. 2023, 14(2), 552–558.
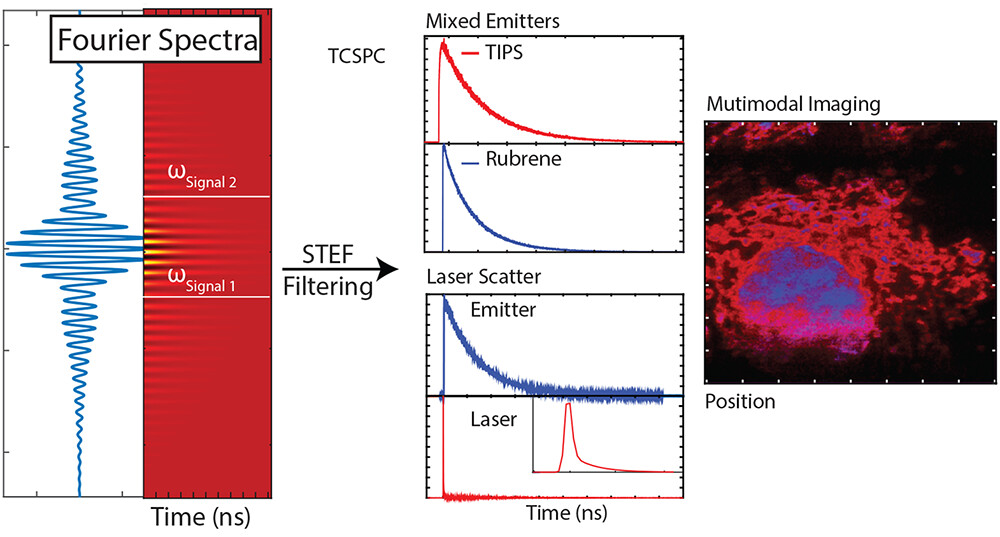
We demonstrate a method for separating and resolving the dynamics of multiple emitters without the use of conventional filters. By directing the photon emission through a fixed path-length imbalanced Mach–Zehnder interferometer, we interferometrically cancel (or enhance) certain spectral signatures corresponding to one emissive species. Our approach, Spectrally selective Time-resolved Emission through Fourier-filtering (STEF), leverages the detection and subtraction of both outputs of a tuned Mach–Zehnder interferometer, which can be combined with time-correlated single photon counting (TCSPC) or confocal imaging to demix multiple emitter signatures. We develop a procedure to calibrate out imperfections in Mach–Zehnder interferometry schemes. Additionally, we demonstrate the range and utility of STEF by performing the following procedures with one measurement: (1) filtering out laser scatter from a sample, (2) separating and measuring a fluorescence lifetime from a binary chromophore mixture with overlapped emission spectra, (3) confocally imaging and separately resolving the standard fluorescent stains in bovine pulmonary endothelial cells and nearly overlapping fluorescent stains on RAW 264.7 cells. This form of spectral balancing can allow for robust and tunable signal sorting.
60. Bailey, A.D.; Deshmukh, A.P.; Bradbury, N.C.; Pengshung, M.; Atallah, T.L.; Williams, J.A.; Barotov, U.; Neuhauser, D.; Sletten, E.M.*; Caram, J.R*. “Exploring the design of superradiant J-aggregates from amphiphilic monomer units.” Nanoscale 2023, 15, 3841–3849.
First posted here: ChemRxiv 2022, DOI: 10.26434/chemrxiv-2021-rjmvz
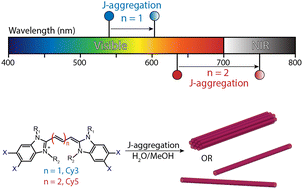
Excitonic chromophore aggregates have wide-ranging applicability in fields such as imaging and energy harvesting; however their rational design requires adapting principles of self-assembly to the requirements of excited state coupling. Using the well-studied amphiphilic cyanine dye C8S3 as a template—known to assemble into tubular excitonic aggregates—we synthesize several redshifted variants and study their self-assembly and photophysics. The new pentamethine dyes retain their tubular self-assembly and demonstrate nearly identical bathochromic shifts and lineshapes well into near-infrared wavelengths. However, detailed photophysical analysis finds that the new aggregates show a significant decline in superradiance. Additionally, cryo-TEM reveals that these aggregates readily form short bundles of nanotubes that have nearly half the radii of their trimethine comparators. We employ computational screening to gain intuition on how the structural components of these new aggregates affect their excitonic states, finding that the narrower tubes are able to assemble into a larger number of arrangements, resulting in more disordered aggregates (i.e. less superradiant) with highly similar degrees of redshift.
2022
59. Estabrook, D.A.; Chapman, J.O.; Yen, S-T.; Lin, H.H.; Ng, E.T.; Zhu, L.; van de Wouw, H.L.; Campàs, O.; Sletten, E.M.* “Macromolecular crowding as an intracellular stimulus for responsive nanomaterials.” J. Am. Chem. Soc. 2022, 144(37), 16792–16798.
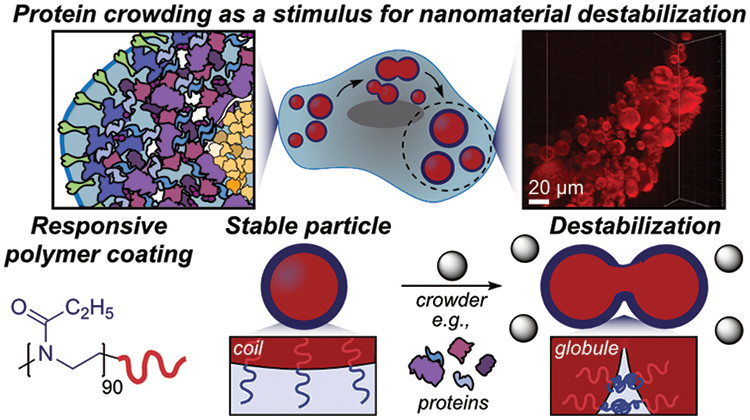
In the field of drug delivery systems, there is a need a new intracellular stimuli for triggering drug release from nanotherapeutics. Here, we explore the use of biomacromolecules as stimuli to trigger the degradation of oil-in-water nanoemulsions. We found that the crowding effect of cytosolic protein concentrations was sufficient to induce fusion of emulsions, through the reduction of polymer lower critical solution temperatures (LCST). When LCST was lowered, emulsions were shown to be responsive to degradation at physiological temperatures. Hence, these thermoresponsive materials were deemed “crowding responsive”. This report sets the stage for classes of thermoresponsive materials to respond to macromolecule concentration rather than temperature changes.
58. Wong, K.C.Y.; Sletten, E.M.* “Extending optical chemical tools and technologies to mice by shifting to the shortwave infrared region.” Curr. Opin. Chem. Biol. 2022, 68, 102131.
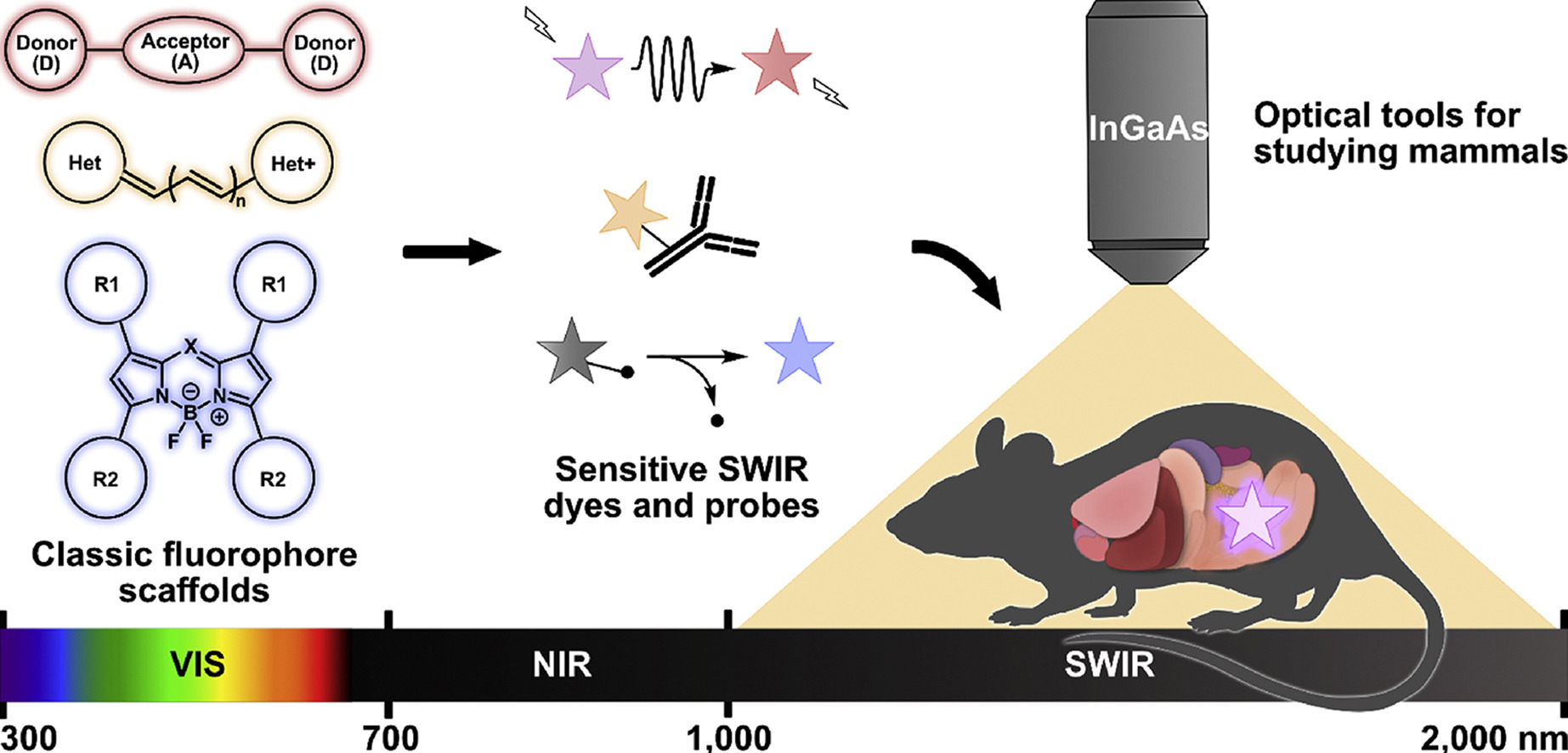
Fluorescence imaging is an indispensable method for studying biological processes non-invasively in cells and transparent organisms. Extension into the shortwave infrared (SWIR, 1000–2000 nm) region of the electromagnetic spectrum has allowed for imaging in mammals with unprecedented depth and resolution for optical imaging. In this review, we summarize recent advances in imaging technologies, dye structure modifications, and use of these dyes as tools for probing biological conditions using SWIR imaging in mice. Finally, we offer an outlook on the future of SWIR detection in the field of chemical biology.
57. Pengshung, M.; Cosco, E.D.; Zhang, Z.; Sletten, E.M.* “Counterion pairing effects on a flavylium heptamethine dye.” Photochem. Photobiol. 2022, 98(2), 303–310.
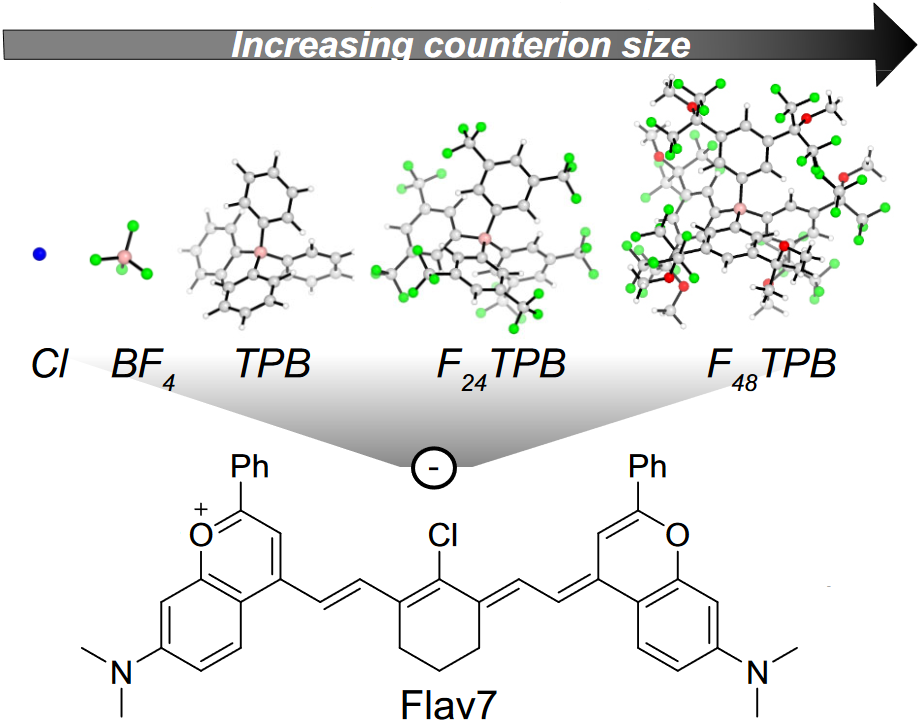
Fluorophores are important tools used in fluorescence imaging. Various renditions of polymethine fluorophores are often used in fluorescence assays due to their superior brightness and tunability of their absorption and emission maxima. However, at high concentrations polymethine dyes are prone to aggregation often making the dye less bright or no longer emissive. Brightness can also be affected by “crossing the cyanine limit” where the dye no longer displays the ideal characteristics of a polymethine dye. Herein, we attempt to overcome these challenges by exchanging the anion (counterion) that is paired with a cationic flavylium heptamethine scaffold. Larger counterions were shown to increase the solubility of the flavylium heptamethine dye in non-polar solvents. Even though counterion effects were subtle with this specific dye, these observations holds potential for further manipulations of these types of polymethine dyes
56. Jia, S.; Sletten, E.M.* “Spatiotemporal control of biology: Synthetic photochemistry toolbox with far-red and near-infrared light.” ACS Chem. Biol. 2022, 17(12), 3255–3269.
This review was published as part of the Young Investigator issue.
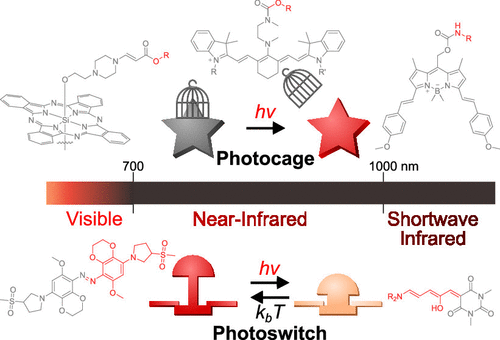
Controlling biological events by light is powerful for the detection and/or alteration of these processes, with wide applications in biological research and strong potential for clinical use. Two of the most widely-studied approaches, reversible photoswitches and irreversible photocages (light-sensitive protection groups), largely rely on photochemistry that occurs under high energy UV or blue light, which exhibits shallow penetration due to the strong absorption and scattering in biological samples. Recently, new chemical designs have expanded these photochemistries to the visible (400-700 nm) and near-infrared (700-1000) regions, with the latter exhibiting deeper penetration depth that enables photochemistries in model animals. This review summarizes current designs of synthetic red and near-infrared photocages and photoswitches with their current and potential biological applications.
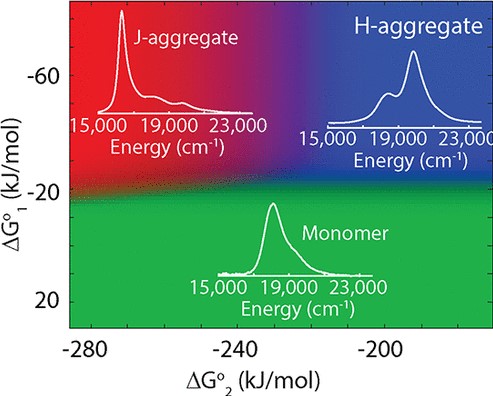
55. Deshmukh, A.; Geue, N.; Bradbury, N.; Atallah, T.L.; Chuang, C.; Pengshung, M.; Cao, J.; Sletten, E.; Neuhauser, D.; Caram, J.* “Bridging the gap between H- and J-Aggregates: Classification and supramolecular tunability for excitonic band structures in 2-dimensional molecular aggregates.“ Chem. Phys. Rev. 2022, 3, 021401.
First posted here: ChemRxiv, 2021, DOI: 10.26434/chemrxiv-2021-ql3b7.
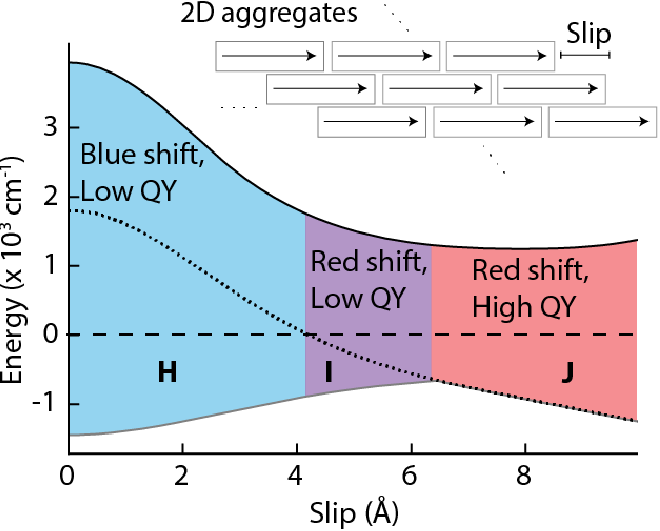
Molecular aggregates with long-range excitonic couplings have drastically different photophysical properties compared to their monomer counterparts. From Kasha’s model for one-dimensional systems, positive or negative excitonic couplings lead to blue or red-shifted optical spectra with respect to the monomers, labeled H-and J-aggregates, respectively. The overall excitonic couplings in higher dimensional systems are much more complicated and cannot be simply classified from their spectral shifts alone. Here, we provide a unified classification for extended 2D aggregates using temperature dependent peak shifts, thermal broadening, and quantum yields. We discuss the examples of six 2D aggregates with J-like absorption spectra but quite drastic changes in quantum yields and superradiance. We find the origin of the differences is, in fact, a different excitonic band structure where the bright state is lower energy than the monomer but still away from the band edge. We call this an “I-aggregate.” Our results provide a description of the complex excitonic behaviors that cannot be explained solely on Kasha’s model. Furthermore, such properties can be tuned with the packing geometries within the aggregates providing supramolecular pathways for controlling them. This will allow for precise optimizations of aggregate properties in their applications across the areas of optoelectronics, photonics, excitonic energy transfer, and shortwave infrared technologies.
2021
54. Jaye, J.; Sletten, E.M.* “Recent advances in the preparation of semifluorinated polymers.” Polym. Chem. 2021, 12, 6515–6526.
This review was published as part of the Early Career Investigator Issue.
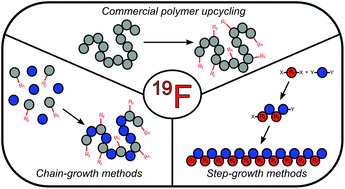
Heavily fluorinated polymers such as Teflon have found widespread commercial and research oriented use due to their unique properties of chemical inertness, superior hydrophobicity, and enhanced thermal stability. Despite these benefits, heavily fluorinated polymers suffer from cumbersome synthesis conditions, low reprocessability, low propensity for forming advanced materials, and notorious bioaccumulation issues. Semifluorinated materials offer potential to have many of the positive properties of their heavily fluorinated analogues, while possessing less of the downsides. This mini-review covers recent advances in the synthesis of semifluorinated polymers, as well as their many applications.
53. Kataki-Anastasakou, A.; Hernandez, S.; Sletten, E.M.* “Cell-surface labeling via bioorthogonal host-guest chemistry.” ACS Chem. Biol. 2021, 16(11), 2124–2129.
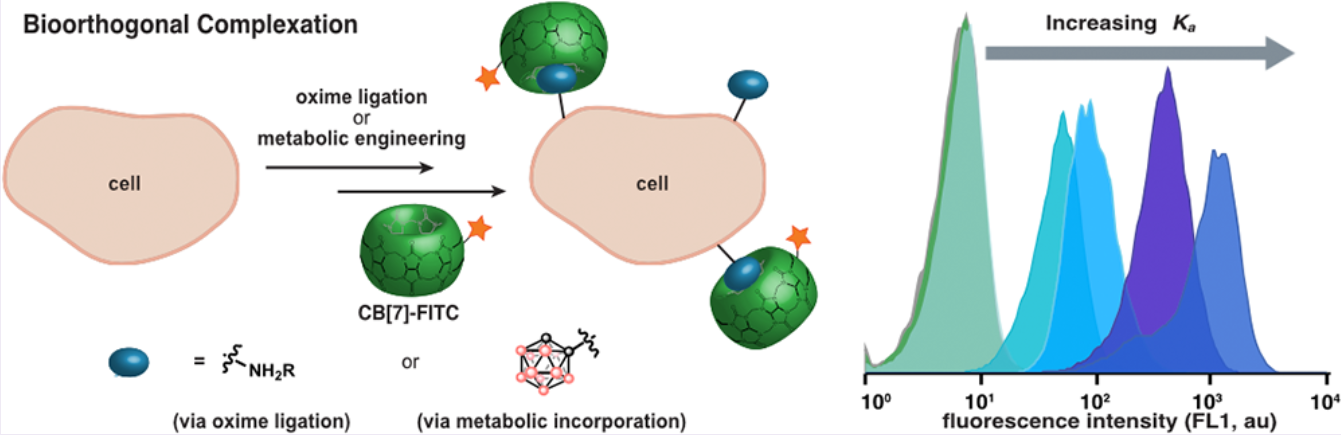
Performing chemistry in living systems, deemed bioorthogonal chemistry, has proven to be
transformational in understanding and manipulating biological processes. Traditional chemistries
rely on the formation of covalent bonds that inside complex organisms may suffer from
incomplete conversions or side-reactivity. Here, we explored the use of molecular recognition or
host-guest chemistry to label cell-surfaces without the need for covalent bond formation. We
introduced unnatural functionality on mammalian cell surfaces and determined how strong the
interaction between guests and a fluorescently-tagged host needs to be to efficiently label cells.
Finally, we were able to metabolically incorporate boron clusters (carboranes) as sialic acid
derivatives onto the cell surface by utilizing the cell’s own biosynthetic machinery. The
carboranes on the cell surface were then non-covalently labelled with a cucurbit[7]uril-
fluorescein conjugate. This work contributes to our understanding of the fundamental
requirements and properties that next-generation host-guest systems must possess to be
translated into complex organisms.
52. Day, R.A.; Sletten, E.M.* “Experimental perspectives on direct visualization of endosomal rupture.” ChemBioChem. 2021, 22(23), 3277–3282.
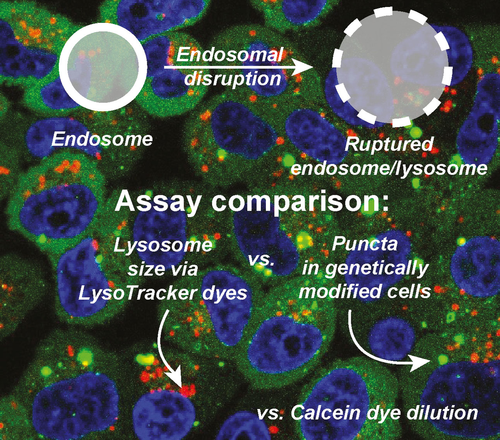
A major limitation of nanomaterials is the inability to efficiently escape the endosome and avoid subsequent degradation. Here, we visualize the disruption of the endosome, separately from the escape of nanomaterials from the endosome. Traditional methods of visualizing endosomal escape focus on the payload. The separation of endosomal membrane disruption from payload release provides insight into this field, with the goal of providing new assays for others working on the problem of endosomal escape.
51. Aguado, B. et al. “35 challenges in materials science being tackled by PIs under 35(ish) in 2021.” Matter 2021, 4, 3804-3810.
50. Friedman, H.C.; Cosco, E.D.; Atallah, T.L.; Jia, S.; Sletten, E.M.; Caram, J.R.* “Establishing design principles for emissive organic SWIR chromophores from energy gap laws.” Chem. 2021, 7(12), 3359–3376.
First posted here: ChemRxiv 2021, DOI: 10.26434/chemrxiv.14374493.v1.
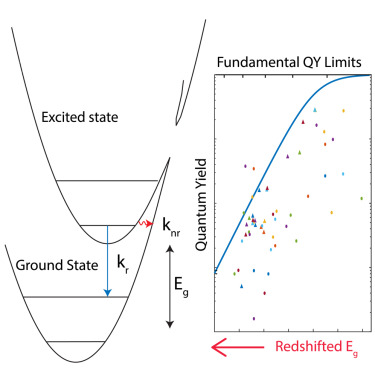
Fluorophores are the key to fluorescence imaging, and one of the key properties is their brightness, which is dependent on the extinction coefficient, describing how strong the fluorophore absorbs light, and the fluorescence quantum yield, evaluating the efficiency of the fluorophore to convert the energy in the light it absorbs to the energy in the light it emits. As researchers design fluorophores with wavelengths as long as 700-1000 nm (near-infrared) and 1000-2000 nm (shortwave infrared) for deep tissue and animal imaging, the fluorescence quantum yield drops precipitously as the wavelength moves longer, limiting the overall brightness of these fluorophores. This phenomenon is explained by the exceeding small energy jump in the excited long-wavelength fluorophore after it absorbs the energy of a photon, or one unit of light. This smaller energy is easier to be lost by the competing pathways of high-frequency molecular vibrations, resulting in heat rather than light being released. Based on experiment and theory, this work establishes an energy gap quantum yield master equation (EQME), which quantifies the limit of fluorescence quantum yield in relationship to the wavelength of a near-infrared or shortwave infrared fluorophore. The equation provides insights in designing brighter near-infrared and shortwave infrared fluorophores and shows the underlying mechanism of quantum yield increase by deuteration or molecular aggregation.
49. Shelton, E.R.*; Kim, S.; Gross, B.J.; Wu, R.; Pochitaloff, M.; Lim, I.; Sletten, E.M.; Campàs, O.* “Stress-driven tissue fluidization physically segments vertebrate somites.” bioRxiv 2021, DOI: 10.1101/2021.03.27.437325.
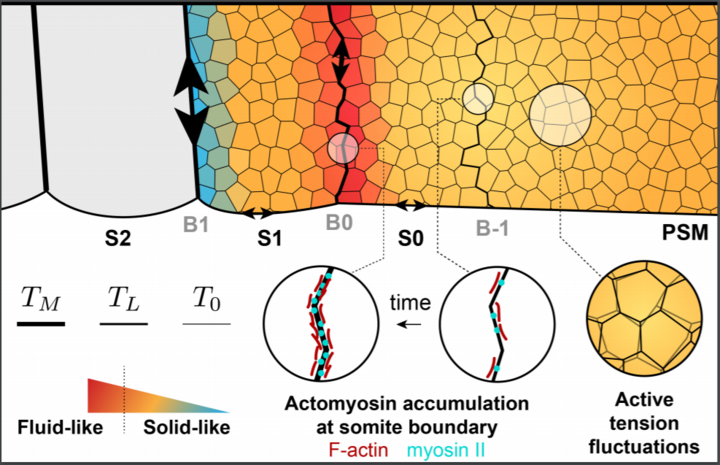
Over the course of embryonic development, functional structures are controlled by both genetic and physical factors. In zebrafish, genetic and molecular signals in the presomitic mesoderm (PSM) orchestrate somite formation. However, the mechanical and physical forces are unelucidated. In this work, direct mechanical measurements, live imaging, and computer simulations are used to explain the segmentation process. Direct mechanical measurements and live imaging are accomplished with a fluorous-soluble Cy5 and perfluorocarbon microdroplets. Overall, actomyosin structure drives an anisotropic stress at nascent somite-somite boundaries, and the tension fluctuations are optimized to define boundaries and minimize morphological defects in the PSM.
48. Jaye, J.A.; Sletten, E.M. “Simple synthesis of fluorinated ene-ynes via in-situ generation of allenes.” Synthesis 2021, 53(22), 4297–4307.
This work was published as part of the special issue in honor of Prof. Sarah Reisman, 2019 Dr. Faul Women in Chemistry Award.
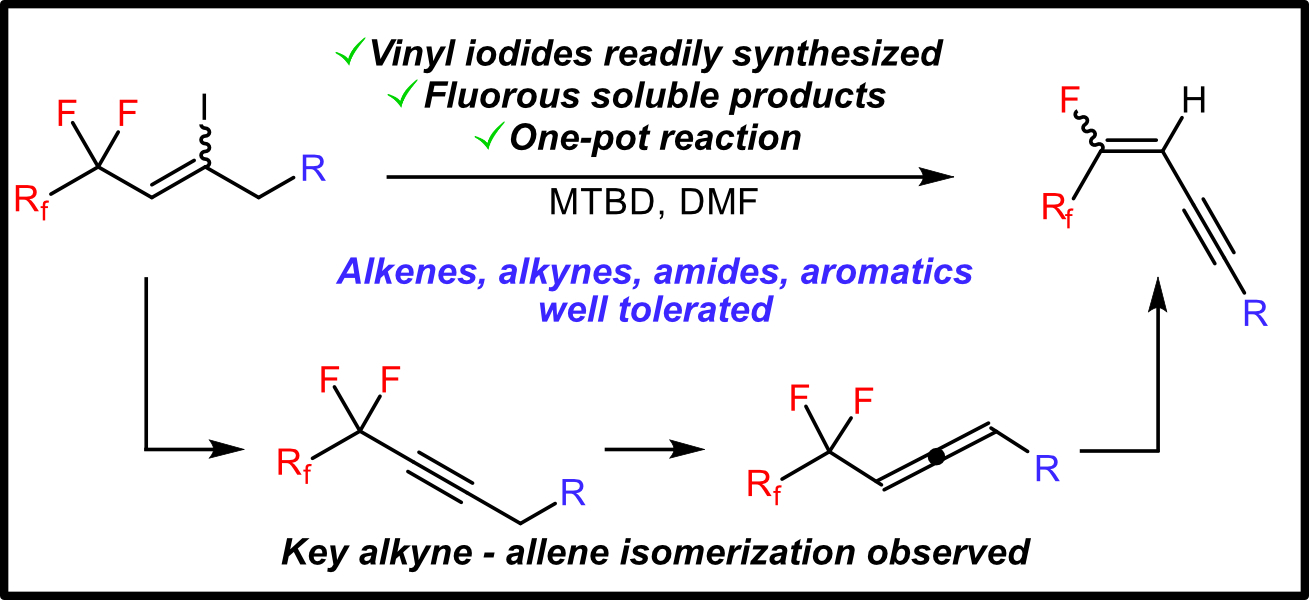
Fluorination of small molecules is a key route toward modulating reactivity and bioactivity. The 1,3 ene-yne functionality is an important synthon towards complex products, as well as a common functionality in biologically active molecules. Here, we present a new synthetic route towards fluorinated ene-ynes through simple starting materials. We employ gas chromatography-mass spectrometry analysis to probe the sequential eliminations necessary for this transformation and observe an allene intermediate. The ene-yne products are sufficiently fluorous to enable purification via fluorous extraction. This methodology will allow facile access to functional, fluorous ene-ynes.
47. Day, R.A.; Sletten, E.M.* “Perfluorocarbon nanomaterials for photodynamic therapy.” Curr. Opin. Colloid Interface Sci. 2021, 54, 101454.
This review was published as part of the Nanobubbles and Nanodroplets issue.
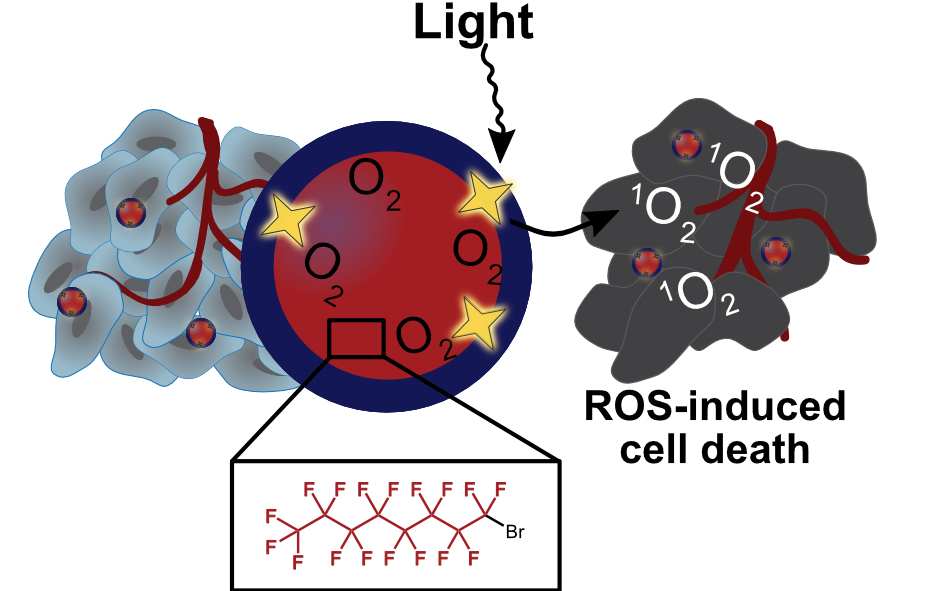
Photodynamic therapy (PDT) is a treatment modality in which a photosensitizer is irradiated with light, producing reactive oxygen species. As it is common for tumors to be hypoxic, methods to deliver photosensitizer and oxygen are desirable. One such approach is the use of perfluorocarbons, molecules in which all C—H bonds are replaced with C—F bonds, to co-deliver oxygen due to the high solubility of gases in perfluorocarbons. This review highlights the benefits and limitations of several fluorinated nanomaterial architectures for use in PDT.
46. Estabrook, D.A.; Day, R.A.; Sletten, E.M.* “Redox‐responsive gene delivery from perfluorocarbon nanoemulsions through cleavable poly(2‐oxazoline) surfactants.” Angew. Chem. Intl. Ed. 2021, 60(32), 17362–17367.
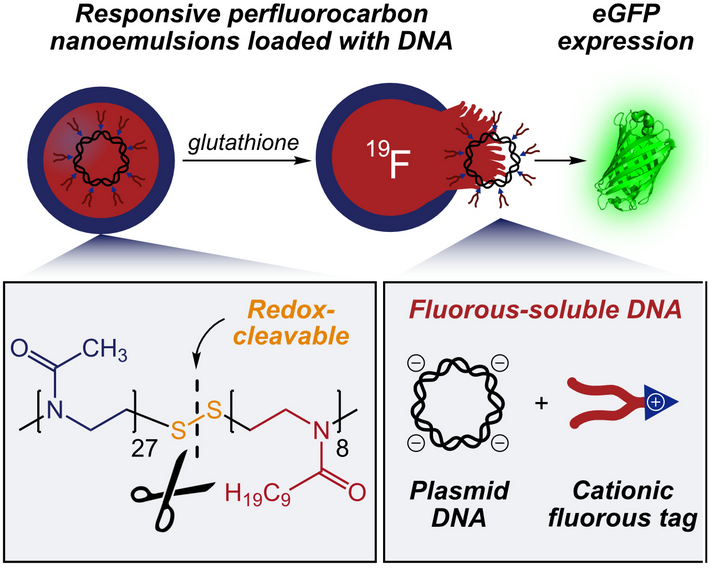
The clinical utility of emulsions as delivery vehicles is hindered by a dependence on passive release. Stimuli-responsive emulsions overcome this limitation but rely on external triggers or are composed of nanoparticle-stabilized droplets that preclude sizes necessary for biomedical applications. Here, we employ cleavable poly(2-oxazoline) diblock copolymer surfactants to form perfluorocarbon (PFC) nanoemulsions that release cargo upon exposure to glutathione. These surfactants allow for the first example of redox-responsive nanoemulsions in cellulo. A noncovalent fluorous tagging strategy is leveraged to solubilize a GFP plasmid inside the PFC nanoemulsions, whereupon protein expression is achieved selectively when employing a stimuli-responsive surfactant. This work contributes a methodology for non-viral gene delivery and represents a general approach to nanoemulsions that respond to endogenous stimuli.
45. Lu, S.; Rodrigues, R.M.; Huang, S.; Estabrook, D.A.; Chapman, J.O.; Guan, X.; Sletten, E.M.; Liu, C.* “Perfluorocarbon nanoemulsions create beneficial O2 microenvironment in N2-fixing biological/inorganic hybrid.” Chem. Catalysis 2021, 1(3), 704–720.
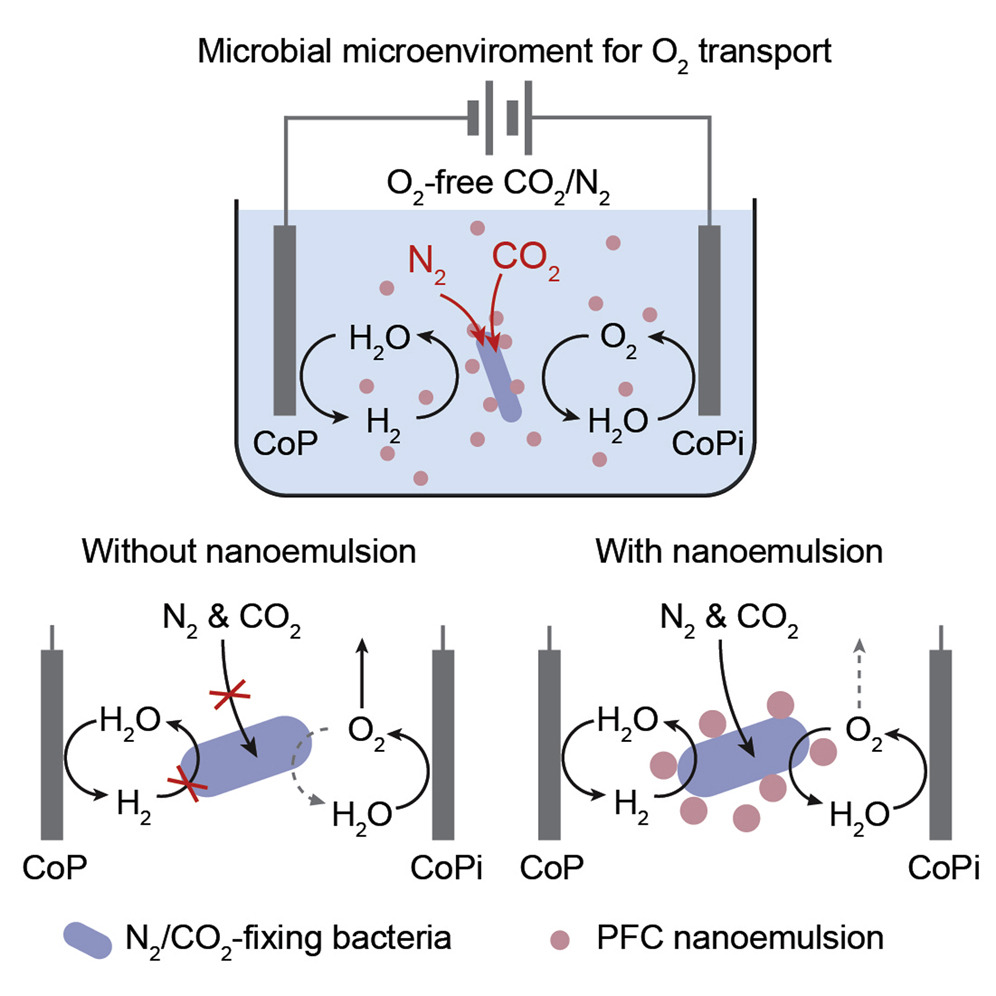
Powered by renewable electricity, biological | inorganic hybrids employ water-splitting electrocatalysis and generate H2 as reducing equivalents for microbial catalysis. The approach integrates the beauty of biocatalysis with the energy efficiency of inorganic materials for sustainable chemical production. Yet a successful integration requires delicate control of the hybrid’s extracellular chemical environment. Such an argument is evident in the exemplary case of O2 because biocatalysis has a stringent requirement of O2, but the electrocatalysis may inadvertently perturb the oxidative pressure of biological moieties. Here we report that the addition of perfluorocarbon nanoemulsions promotes a biocompatible O2 microenvironment in an O2-sensitive N2-fixing biological | inorganic hybrid. Langmuir-type nonspecific binding between bacteria and nanoemulsions facilitates O2 transport in a bacterial microenvironment and leads to a 250% increase in efficiency for organic fertilizers within 120 h. Controlling the biological microenvironment with nanomaterials heralds a general approach accommodating the compatibility in biological | inorganic hybrids.
44. Cosco, E.D.; Lim, I.; Sletten, E.M.* “Photophysical properties of indocyanine green in the shortwave infrared region.” ChemPhotoChem 2021, 5(8), 727–734.
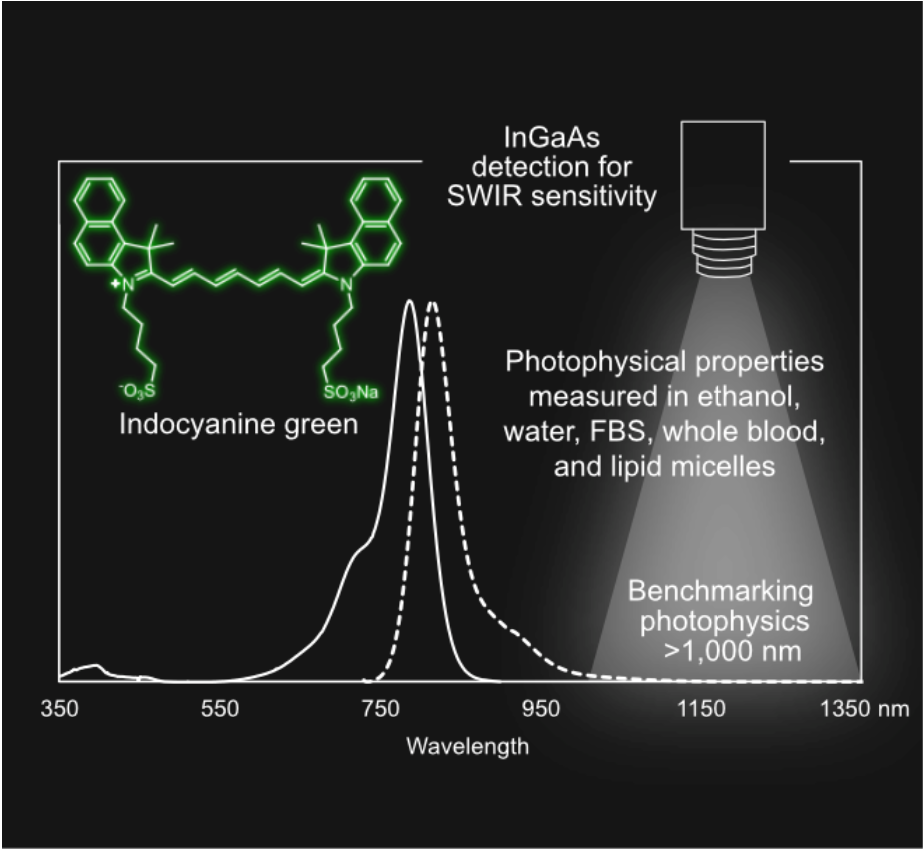
With the growing development of new contrast agents for optical imaging using near‐infrared and shortwave infrared (SWIR) wavelengths, it is essential to have consistent benchmarks for emitters in these regions. Indocyanine green (ICG), a ubiquitous and FDA approved organic dye and optical imaging agent, is commonly employed as a standard for photophysical properties and biological performance for imaging experiments at these wavelengths. Yet, its reported photophysical properties across organic and aqueous solvents vary greatly in the literature, which hinders its ability to be used as a consistent benchmark. Here, we measure photophysical properties in organic and aqueous solvents using InGaAs detection (~950–1700 nm), providing particular relevance for SWIR imaging.
43. Lee, G.Y.; Hu, E.; Rheingold, A.L.; Houk, K.N.*; Sletten, E.M.* “Arene-perfluoroarene interactions in solution.” J. Org. Chem. 2021, 86(12), 8425–8436.
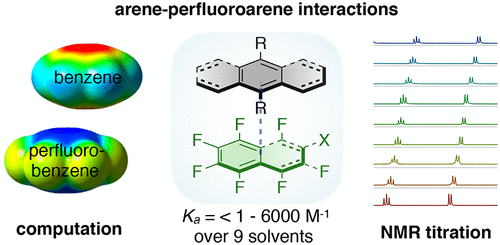
Non-covalent interactions involving aromatic rings are responsible for a wide array of phenomena in chemistry and biology. An interesting and yet underexplored non-covalent interaction is arene-perfluoroarene interaction. Here, we report a systematic study of arene-perfluoroarene interactions in solution. Using a combination of NMR titration experiments, X-ray crystallography, and computational analysis, we analyze the effects of fluorination, substituents, ring size, and solvation on the arene-perfluoroarene interaction. We find that fluorination, extension of the π systems, and enhancement of solvent polarity greatly stabilize the stacking energy up to 3 orders of magnitude (Ka = <1 to 6000 M–1), with the highest Ka achieved in buffered D2O (pD = 12). The enhanced understanding of arene-perfluoroarene interactions in aqueous solution sets the stage for the implementation of this abiotic intermolecular interaction in biology and medicine.
42. Cosco, E.D.; Arus, B.A.; Spearman, A.L.; Atallah, T.L.; Lim, I.; Leland, O.S.; Caram, J.R.; Bischof, T.S. Bruns, O.T.*; Sletten, E.M.* “Bright chromenylium polymethine dyes enable fast, four-color in vivo imaging with shortwave infrared detection.” J. Am. Chem. Soc. 2021, 143(18), 6836–6846.
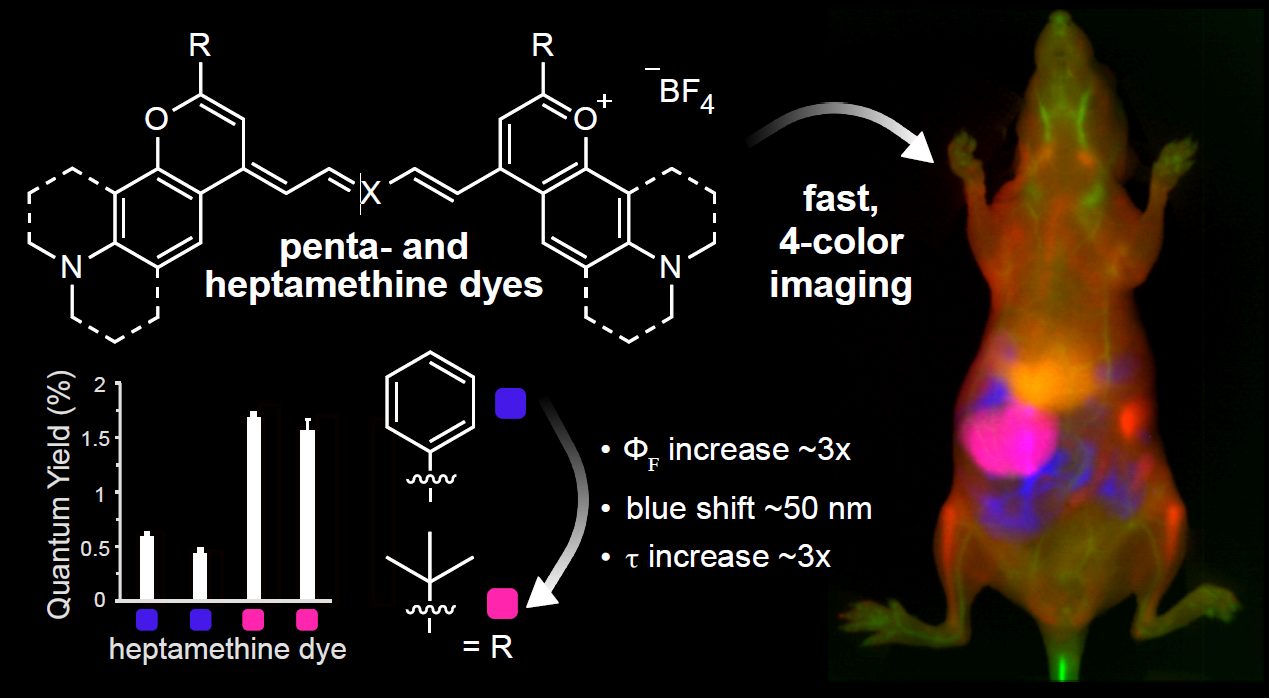
Fluorescence imaging in the shortwave infrared (SWIR, 1000–2000 nm) region of the electromagnetic spectrum enables non-invasive, high-resolution and high-contrast in vivo imaging. With orthogonally excited fluorophores, multiplexed imaging allows visualization of multiple biological parameters in real time. Polymethine dyes are optimal probes for SWIR multiplexed imaging, thanks to their high absorption coefficients and narrow absorption spectra. This work improves upon previous multiplexed imaging efforts with the synthesis of bright chromenylium-based polymethine dyes matched to common laser lines. These chromenylium dyes are based on 2-position modifications to the flavylium scaffold and have significantly higher quantum yields due to decreased non-radiative rates. This work has enabled non-invasive single-color imaging at 300 fps and 3-color imaging at 100 fps: the fastest SWIR imaging to date. Finally, in concert with previously reported dyes, we achieved 4-color video-rate in vivo SWIR imaging for the first time. We envision that the development of brighter dyes for SWIR multiplexed imaging will lead to improved medical imaging technologies.
2020
41. Mu X.; Hopp, M.; Dziedzic, R.M.; Rheingold, A.L.; Sletten, E.M.; Axtell, J.C.*; Spokoyny, A.M.* “Expanding the scope of palladium-catalyzed B—N cross-coupling chemistry in carboranes.” Organometallics 2020, 39(23), 4380–4386.
First posted here: ChemRxiv 2020, DOI: 10.26434/chemrxiv.12844820.v1
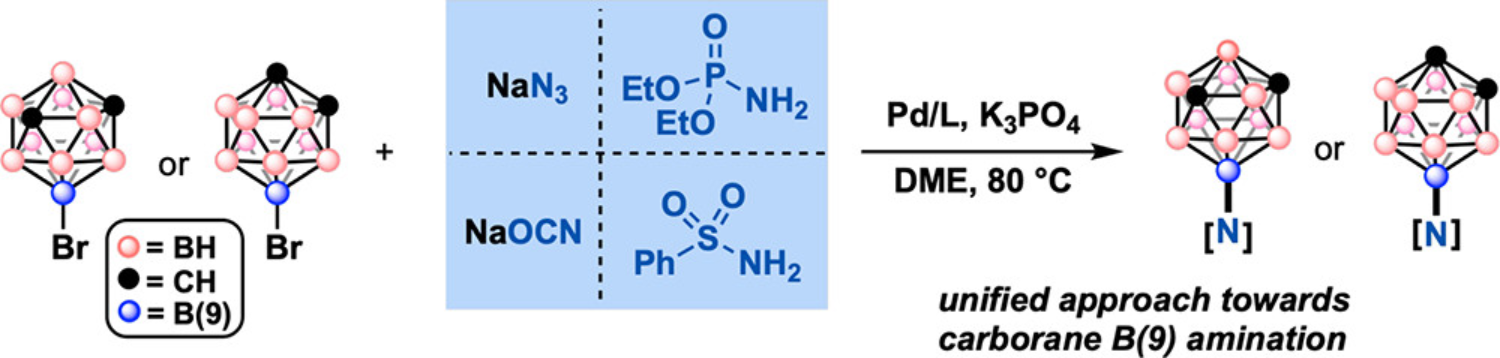
Our interest in host-guest chemistry and its potential for introducing new developments in the field of chemical biology has prompted a closer look into promising candidates for both components. Carboranes — polyhedral boron clusters composed of catenated C-H and B-H vertices — exist in various shapes and charges and have increasingly been applied in chemical research due to their topology and electronic properties. We recently identified these molecules as promising guest targets and demonstrated their utility for the recycling of cucurbit[7]uril hosts, made possible through new amination chemistry on the carborane cage. In this paper, given the traditional difficulty in forging B-N bonds on certain carborane vertices, we opted to explore the breath of this synthetic strategy with two carborane substrates. We find that several nitrogen-based coupling partners — including phosphoramidates and isocyanates — can be rapidly installed, providing versatile, reactive handles for future chemistry.
40. Kataki-Anastasakou, A.; Axtell, J.C.; Hernandez, S.; Dziedzic, R.M.; Balaich, G.J.; Rheingold, A.L.; Spokoyny, A.M.; Sletten, E.M.* “Carborane guests for cucurbit[7]uril facilitate strong binding and on demand removal.” J. Am. Chem. Soc. 2020, 142(49), 20513–20518.
First posted here: ChemRxiv 2020, DOI: 10.26434/chemrxiv.12844820.v1
Highlighted in Synfacts
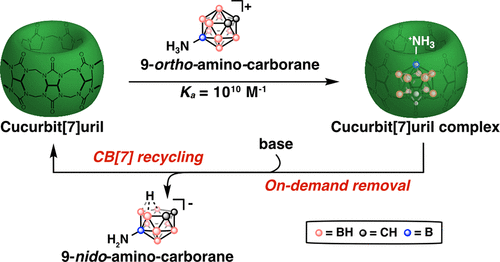
Cucurbit[7]uril (CB[7]) has become the host of choice in biomolecule purification, small molecule separation, polymer cross-linking and sensor development. Previously discovered strong binding guests for CB[7] have been difficult to remove from the CB[7] cavity thus limiting widespread adoption of host-guest chemistry applications. In this paper, we introduce a new class of CB[7] guests, namely ortho-carboranes, that can be removed on demand using a mild chemical trigger. Water soluble derivatives of ortho-carborane and their isomers meta-carboranes were synthesized and shown to have high binding affinity to CB[7] as well as orthogonal response to “deboronation” that causes meta-carboranes to be stable against removal. Finally, in a proof-of-concept study a CB[7]-decorated resin was used to isolate a fluorescent payload and amino-ortho-carborane was used to release the payload and recycle the CB[7]-resin through on-demand removal.
39. Estabrook, D.A.; Sletten, E.M.* “Printing precise materials with visible light.” ACS. Cent. Sci. 2020, 6(9), 1482–1484.
A First Reactions piece for: “Rapid High-Resolution Visible Light 3D Printing“
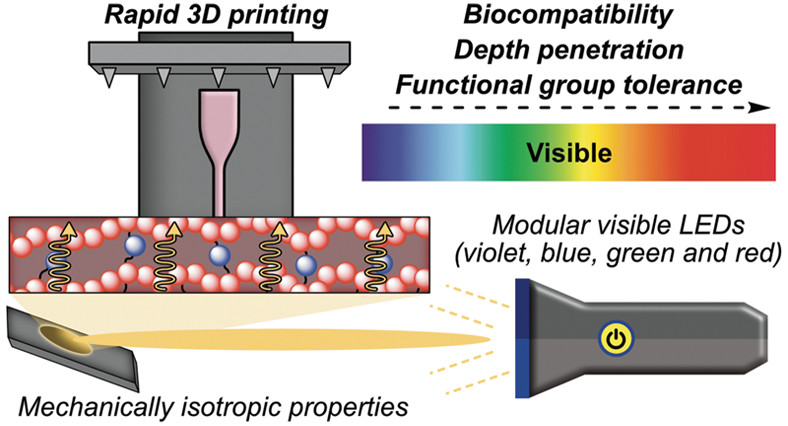
A three-component system uses four different wavelengths of visible light to rapidly print 3D materials with versatile mechanical properties and precise feature sizes.
38. Lim, I.; Vian, A.; van de Wouw, H.L.; Day, R.A.; Gomez, C.; Liu, Y.; Rheingold, A.L.; Campàs, O.; Sletten, E.M.* “Fluorous soluble cyanine dyes for visualizing perfluorocarbons in living systems.” J. Am. Chem. Soc. 2020, 142(37), 16072–16081.
Highlighted in JACS Spotlights
Included in the 2021 Early Career Investigator Issue of JACS
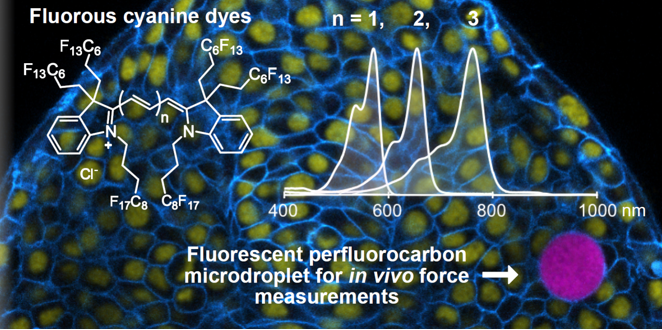
Perfluorocarbon emulsions have been used in biological settings as oxygen delivery vehicles, ultrasound contrast agents, and F-MRI contrast agents. In this paper, we enable the the fluorescence visualization of perfluorocarbon emulsions by synthesizing fluorous-soluble cyanine dyes. These dyes were photophysically characterized in fluorous solvent, which few fluorous-soluble fluorophores have done. We also showed the fluorous solubility of these cyanine dyes were superior to a benchmark rhodamine. We used perfluorocarbon nanoemulsions labelled with a red-shifted cyanine to perform multichannel microscopy in cells. Larger droplets labelled with the same cyanine yielded biophysical force information in zebrafish and multicellular aggregate models.
37. Deshmukh, A.P.; Bailey, A.D.; Forte, L.S.; Shen, X.; Geue, N.; Sletten, E.M.; Caram, J.R.* “Thermodynamic control over molecular aggregate assembly enables tunable excitonic properties across the visible and near-infrared.” J. Phys. Chem. Lett. 2020, 11(19), 8026–8033.
Specific molecular arrangements within H-/J-aggregates of cyanine dyes enable extraordinary photophysical properties, including long-range exciton delocalization, extreme blue/red shifts, and excitonic superradiance. Despite extensive literature on cyanine aggregates, design principles that drive the self-assembly to a preferred H- or J-aggregated state are unknown. We tune the thermodynamics of self-assembly via independent control of the solvent/nonsolvent ratio, ionic strength, or dye concentration, obtaining a broad range of conditions that predictably stabilize the monomer (H-/J-aggregate). Diffusion-ordered spectroscopy, cryo-electron microscopy, and atomic force microscopy together reveal a dynamic equilibrium between monomers, H-aggregated dimers, and extended J-aggregated 2D monolayers. We construct a model that predicts the equilibrium composition for a range of standard Gibbs free energies, providing a vast aggregation space which we access using the aforementioned solvation factors. We demonstrate the universality of this approach among several sheet-forming cyanine dyes with tunable absorptions spanning visible, near, and shortwave infrared wavelengths.
36. Pengshung, M.; Li, J.; Mukadum, F.; Lopez, S.A.*; Sletten, E.M.* “Photophysical tuning of shortwave infrared flavylium heptamethine dyes via substituent placement.” Org. Lett. 2020, 22(15), 6150–6154.
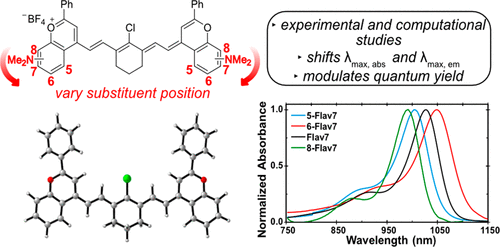
Fluorophores in the shortwave infrared region (SWIR, 1000-2000 nm) of the electromagnetic spectrum have gained a lot of interest recently for fluorescence imaging. However, more often than not these fluorophores are not biocompatible or are very dim. We have previously developed a small molecule dye, Flav7, with acceptable brightness in the SWIR. In this article, we explore structural-property relationship of flavylium heptamethines by changing the position of the dimethylamino substituent around the ring. Through computational and experimental analysis, we explore how these positions effect photophysical properties and gain a deeper understanding on how to design fluorophores for the SWIR.
35. Day, R.D.; Estabrook, D.A.; Wu, C.; Chapman, J.O.; Togle, A.; Sletten, E.M.* “Systematic study of perfluorocarbon nanoemulsions stabilized by polymer amphiphiles.” ACS Appl. Mater. Interfaces 2020, 12(35), 38887–38898.
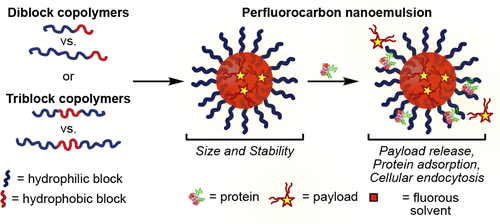
Perfluorocarbon nanoemulsions, droplets of fluorous solvent stabilized in water through a surfactant are a modular nanomaterial for the treatment and diagnosis of disease. In this work, we systematically vary the structure of the surfactant in order to understand the structure-property relationship. For each surfactant we analyze the size, stability, payload retention, cellular uptake and protein adsorption on perfluorocarbon nanoemulsions. We find the hydrophilic block length and identity, polymer hydrophilic: lipophilic balance, and polymer architecture are important parameters when selecting a surfactant to stabilize emulsions.
34. Cosco, E.D.; Spearman, A.L.; Ramakrishnan, S.; Lingg, J.G.P.; Saccomano, M.; Pengshung, M.; Arus, B.A.; Wong, K.C.Y.; Glasl, S.; Ntziachristos, V.; Warmer, M.; McLaughlin, R.R.; Bruns, O.T.*; Sletten, E.M.* “Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time.” Nat. Chem. 2020, 12, 1123–1130.
Learn more about Dr. Maly Cosco’s experience while working on this project: https://chemistrycommunity.nature.com/posts/multiplexing-in-mice-joining-chemistry-and-imaging-across-continents
Featured in Phys.org
Highlighted by Burgess and coworkers in ChemPhotoChem
Highlighted by the Daily Bruin
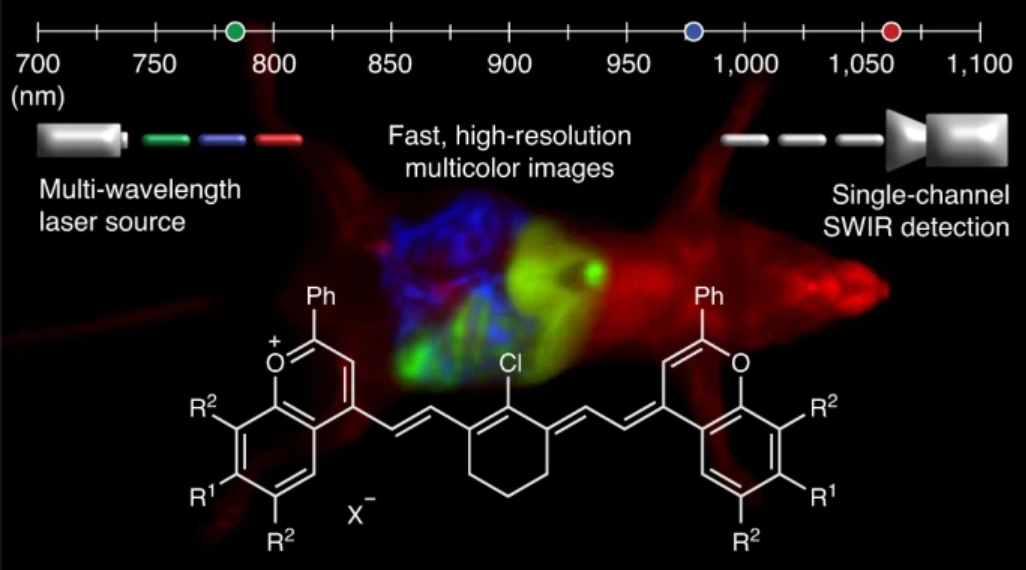
High-resolution, in vivo multiplexed imaging has been achieved in cellulo but translation to mammals has been a challenge. Existing fluorophores in the visible (350-700 nm) and near infrared (700-1000 nm) regions can suffer low tissue penetration and high background autofluorescence respectively. The shortwave infrared region (SWIR, 1000-2000 nm) avoids these limitations and is therefore suitable for real-time, multiplexed imaging in animals. Through the elucidation of flavylium polymethine structure-property relationships, we were able to design SWIR fluorophores matched to common laser lines (980 and 1064 nm). We then developed an imaging system with variable near-infrared/SWIR excitation and single-channel detection, allowing for video-rate multicolor SWIR imaging for optically guided surgery and imaging of awake and moving mice with multiplexed detection. This work enables real time monitoring of orthogonal functions in unrestrained moving animals. Additionally, the development of polymethine-based SWIR probes as well as clinical SWIR imaging systems will lead to improved surgical, diagnostic, and biomedical studies.
33. Miller, M.A.; Sletten, E.M.* “Perfluorocarbons in Chemical Biology.” ChemBioChem 2020, 21(24), 3451–3462.
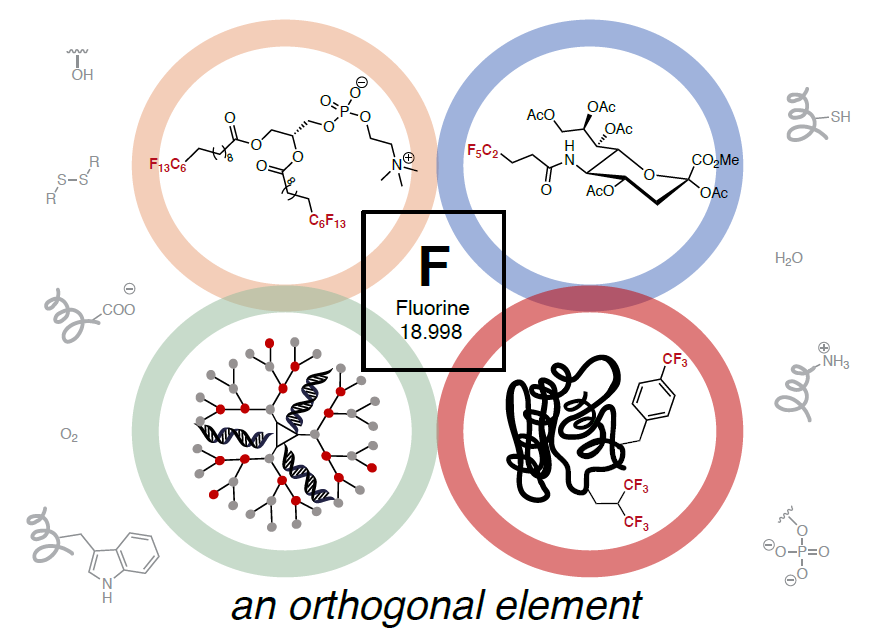
Chemical biology is a field that uses chemical tools to study and manipulate biology. One way this is done is to introduce unnatural groups that can be detected among all the naturally-occurring small molecules and biomolecules. Perfluorocarbons are carbon chains where all the hydrogen atoms have been replaced with fluorine atoms. These molecules are not found in mammalian tissue and can be considered “orthogonal” to many living systems. In this review, we discuss how perfluorinated unnatural groups have been incorporated into and interact with biomolecules as a way to study biological systems.
32. Pengshung, M.; Neal, P.; Atallah, T.L.; Kwon, J.; Caram, J.R.*; Lopez, S.A.*; Sletten, E.M.* “Silicon incorporation in polymethine dyes.” Chem. Commun. 2020, 56, 6110–6113.
First posted here: ChemRxiv 2019, DOI: 10.26434/chemrxiv.11320064.v1.
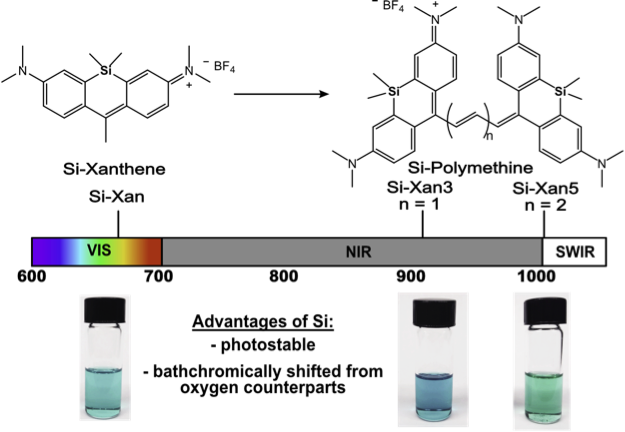
Imaging in complex biological systems is difficult due to the limitation of current fluorescent probes. To overcome this, we are focused on developing dyes with red-shifted (longer) absorption and emission wavelengths. Herein we have shown the first example of incorporating silicon into polymethines which red-shifts 100 nm and increases photostability. This showcases how simple structural modifications to molecules can cause significant photophysical changes that can be used in the design of future probes.
31. Jaye, J.A.; Sletten, E.M.* “Vinyl iodide containing polymers directly prepared via an iodo-yne polymerization.” ACS Macro Lett. 2020, 9(3), 410–415.
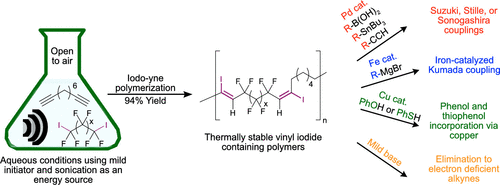
The vinyl halide functionality is ubiquitous in small molecule chemistry, but examples of polymer containing vinyl halides are rare. We have synthesized vinyl iodide containing fluorinated polymers through reaction of diynes and diiodoperfluoroalkanes. These polymers could undergo multiple cross-couplings reactions to alter thermal and physical properties. Vinyl iodide could also be eliminated to generate activated alkynes which can undergo cycloaddition chemistry. We expect these polymers to be used for applications which benefit from an array of post-polymerization modifications.
30. Miller, M.A.; Day, R.D.; Estabrook, D.A.; Sletten, E.M.* “A reduction-sensitive fluorous fluorogenic coumarin.” Synlett 2020, 31(05), 450–454.
Published as part of the Special Section for the 11th EuCheMS Organic Division Young Investigator Workshop.
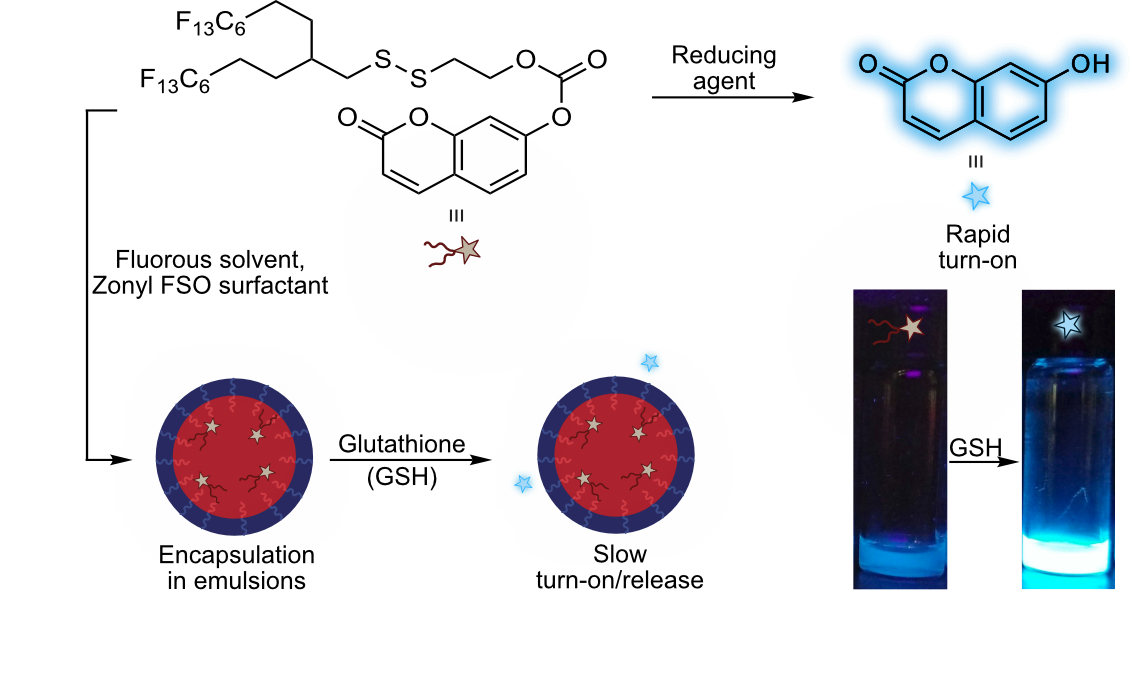
Dyes sensitive to their environment are useful tools for sensing chemical changes and probing biological systems. Traditionally, these probes have been developed for use in organic solvents, aqueous buffers, or the gas phase, with little attention paid to their utility in the fluorous phase. Herein, we synthesized a fluorous-soluble dye that lights up when exposed to a biologically relevant reducing agent. We expect that these types of dyes will find use in the expanding fluorous drug delivery community.
2019
29. Chen, W.; Cheng, C.-A.; Cosco, E.D.; Ramakrishnan, S.; Lingg, J.G.P.; Bruns, O.T.*; Zink, J.I.*; Sletten, E.M.* “Shortwave infrared imaging with J-aggregates stabilized in hollow mesoporous silica nanoparticles.” J. Am. Chem. Soc. 2019, 141(32), 12475–12480.
First posted here: ChemRxiv 2018, DOI: 10.26434/chemrxiv.7503506.v1.
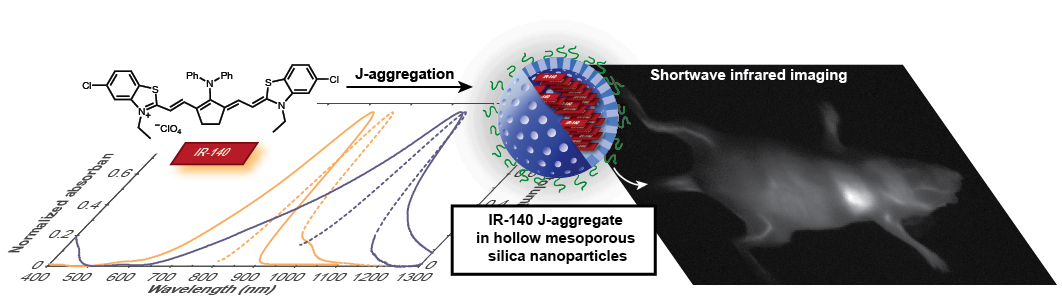
Organic chromophores are known to be bright and biocompatible agents for optical imaging using visible and near-infrared light. Recently, we and others have focused on tuning molecular structure of polymethine dyes to red-shift monomer absorption wavelengths into the shortwave infrared (SWIR), where tissue properties are more favorable for imaging in mammals. However, this pursuit comes with challenges in brightness and stability for long-wavelength absorbing monomers. Here, we explore J-aggregation as a new strategy to create biocompatible polymethine-loaded nanoparticle imaging agents which absorb and emit SWIR light.
28. Jaye, J.A.; Sletten, E.M.* “Modular and processable fluoropolymers prepared via a safe, mild, iodo-ene polymerization.” ACS Cent. Sci. 2019, 5(6), 982–991.
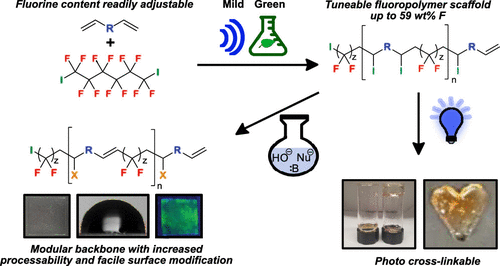
Commercial fluorinated polymers are developed for many applications but can suffer from processability issues and are difficult to derivatize. We have developed new methodology to generate high molecular weight fluoropolymers with a modular backbone dependent on diene functionality. We also demonstrate multiple post-polymerization modifications to place azide, thiol, and allyl functionalities across the polymer. Irradiation with UV light and an initiator allowed facile cross-linking into fluorinated gels. We expect these polymers to lead to new commercial applications.
27. Estabrook, D.A.; Ennis, A.F.; Day, R.A.; Sletten, E.M.* “Controlling nanoemulsion surface chemistry with poly(2-oxazoline) amphiphiles.” Chem. Sci. 2019, 10, 3994–4003.
First posted here: ChemRxiv 2018, DOI: 10.26434/chemrxiv.7052027.
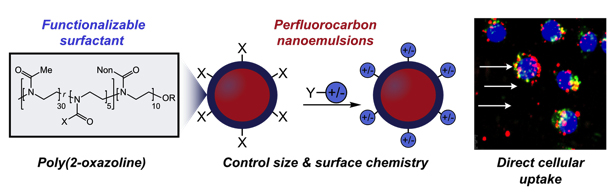
Emulsions are liquid-in-liquid droplets that are found in the pharmaceutical, food and cosmetic industries. However, use of these droplets is limited by challenges in controlling their properties, like size, charge or surface chemistry. In this paper, we make functional macromolecules (i.e. polymers) that self-assemble at the surface of these droplets; thus, by controlling the polymer, we can control resulting droplet properties on the nanoscale. Further, we show that particular properties dictate how these droplets behave in biological environments, demonstrating the importance of these parameters in real-world applications.
26. Rodrigues, R.M.; Guan, X.; Iniguez, J.A.; Estabrook, D.A.; Chapman, J.O.; Huang, S.; Sletten, E.M.; Liu, C.* “Perfluorocarbon nanoemulsion promotes the delivery of reducing equivalents for electricitry-driven microbial CO2 reduction.” Nature Catalysis 2019, 2, 4017–4014.
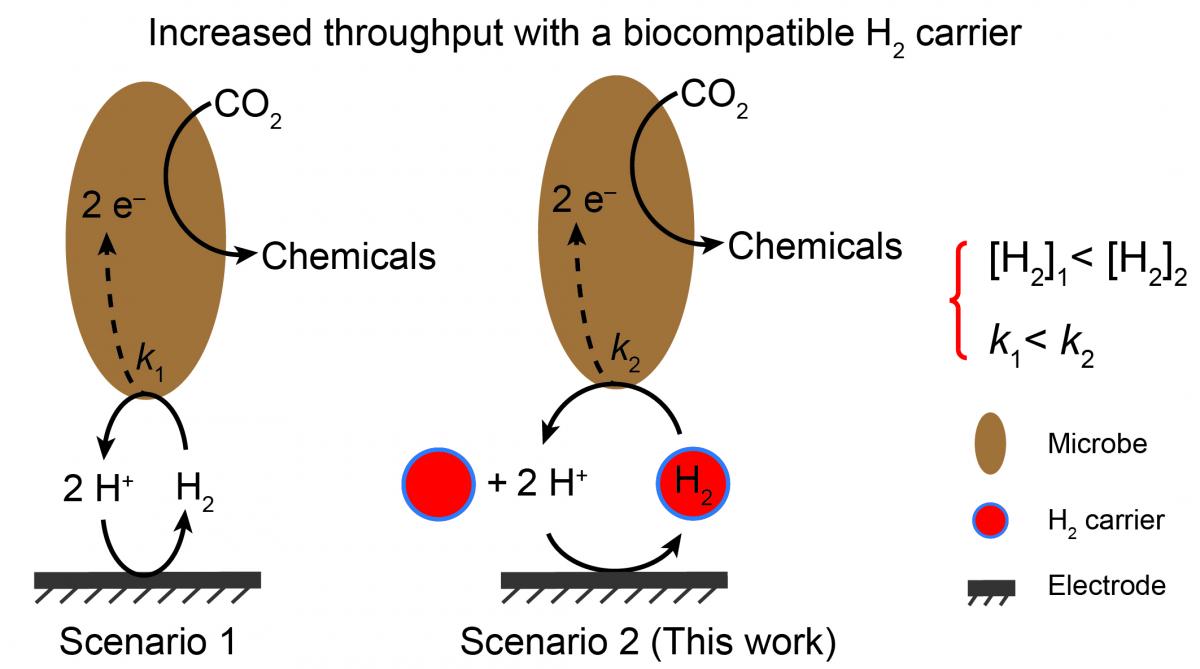
The reduction of CO2 into chemicals and fuels is a promising way to transform and store renewable energies while removing greenhouse gases. Previously, Prof. Chong Liu has integrated inorganic electrochemical catalysts with CO2-fixing microorganisms in order to convert CO2 gas into acetic acid, a high-value chemical. However, the maximum throughput was limited by the solubility and transfer kinetics of H2 gas involved within the pathway. In this collaboration, we demonstrated that perfluorocarbon nanoemulsions–known gas carriers–can work to promote microbial CO2 reduction through efficient H2 delivery. The design principles described herein show how fluorous nanocarriers can impact the way we approach converting greenhouse gases into commodity chemicals, and are expected to be applicable to other processes (e.g. N2 fixation and CH4 functionalization).
2018
25. Cao, W.; Sletten, E.M.* “Fluorescent cyanine dye J-aggregates in the fluorous phase.” J. Am. Chem. Soc. 2018, 140(8), 2727–2730.
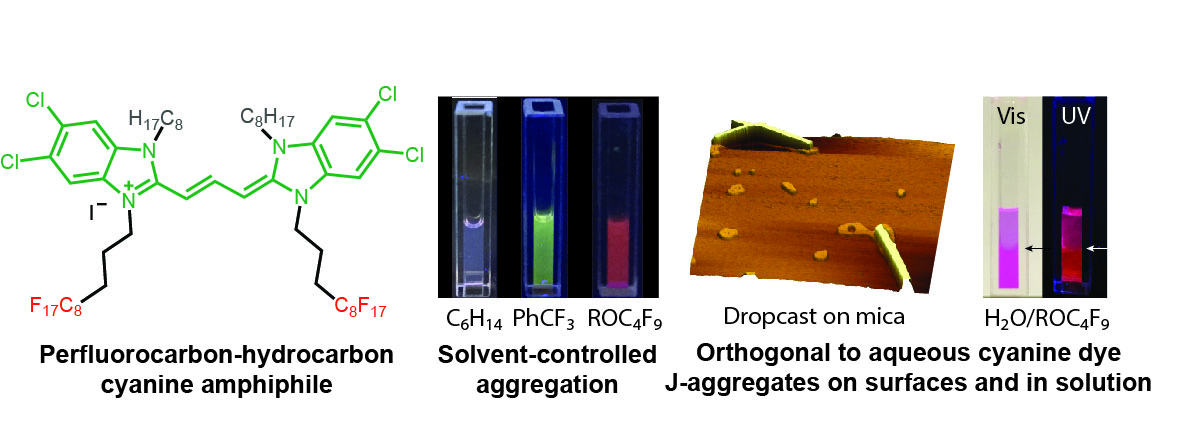
J-aggregates are a unique fluorophore formation that allows for enhanced photophysical properties, such as red-shifted absorption/emission and increased brightness. Thus, it is of interest to be able to utilize these aggregates for a wide variety of different applications. Traditionally J-aggregates are formed by cyanines in aqueous solutions which severely limits their processability. Herein, we develop a perfluorocarbon-hydrocarbon amphiphilic cyanine dye that J-aggregates in nonaqueous media. This fluorous J-aggregate showcases enhanced photostability and ease of fabrication in comparison to traditional cyanine aggregates, making them more readily applicable to future technologies.
24. Miller, M.A.; Sletten, E.M.* “A general approach to biocompatible branched fluorous tags for increased solubility in perfluorocarbon solvents.” Org. Lett. 2018, 20(21), 6850–6854.
Highlighted in Synfacts
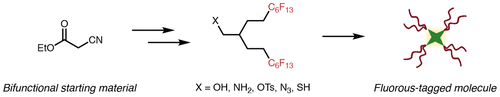
Molecules with many C-F bonds are referred to as perfluorocarbons. These compounds have many useful properties, however long-chain, linear perfluorocarbons (ex. perfluorooctanoic acid, PFOA) persist in the environment and are not readily broken down in the body. In this paper, we have developed a simple method to synthesize branched, short-chain fluorinated tags. These tags retain the properties of highly fluorinated compounds so that perfluorocarbons can be applied in a more biocompatible manner.
2017
23. Day, R.A.; Estabrook, D.A.; Logan, J.K.; Sletten, E.M.* “Fluorous photosensitizers enhance photodynamic therapy with perfluorocarbon nanoemulsions.” Chem. Commun. 2017, 53, 13043–13046.
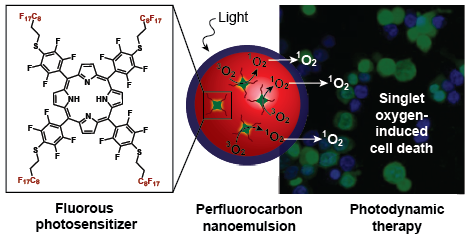
Photodynamic therapy – a treatment that uses light, oxygen, and a small molecule photosensitizer to produce toxic reactive oxygen species – has been utilized successfully to treat actinic keratosis, small cell carcinoma, pleural mesothelioma, oesophageal, non-small cell lung and skin cancer. Previous efforts to increase photodynamic efficiency have focused on the development of new photosensitizers. In this work, we delivered the photosensitizer and oxygen simultaneously to increase the amount of reactive oxygen species produced when irradiated with light. Through the co-delivery of oxygen, photodynamic therapy can now be a viable treatment method for diseases (such as solid tumors) that are often lacking sufficient oxygen.
22. Cosco, E.D.; Caram, J.R.; Bruns, O.T.; Franke, D.; Day, R.A.; Farr, E.P.; Bawendi, M.G.; Sletten, E.M.* “Flavylium polymethine fluorophores for near- and shortwave infrared imaging.” Angew. Chem. Int. Ed. 2017, 56(42), 13126–13129.
Highlighted in Nature and ChemistryViews
Optical imaging with shortwave infrared detection (SWIR, 1000–2000 nm) offers superior contrast, resolution and depth penetration compared with near infrared or visible light detection. While the first contrast agents used for SWIR imaging included carbon nanotubes, rare-earth metal composites, and quantum dots, bright small molecule fluorophores would enable increased biocompatibility and translation of this technology to the clinic. Creating bright molecules which absorb and emit light in SWIR region of the electromagnetic spectrum is a challenging problem for organic chemists. In this study, we designed long wavelength analogues of a molecular scaffold of fluorescent dyes, called cyanine dyes, which are commonly employed with visible and near-infrared wavelengths of light. The result was a series of polymethine dyes which were about 200 nm red shifted from the traditional cyanine dyes. The compound containing 7 methine units in its linker absorbs and emits SWIR light and became the first polymethine dye designed for SWIR imaging. Notably, it also represented the brightest SWIR-light absorbing small molecule dye reported so far. We applied this dye to image deep vasculature in mice.
Postdoctoral Work
21. Sletten, E.M.; Swager, T.M. “Readily accessible multifunctional fluorous emulsions.” Chem. Sci. 2016, 7, 5091–5097.
20. Niroui, F.; Wang, A.I.; Sletten, E.M.; Song, Y.; Kong, J.; Yablonovitch, E.; Swager, T.M.; Lang, J.H.; Bulovic, V. “Tunneling nanoelectromechanical switches based on compressible molecular thin films.” ACS Nano 2015, 9, 7886–7894.
19. Zarzar, L.D.; Sresht, V.; Sletten, E.M.; Kalow, J.A.; Blankschtein, D.; Swager, T.M. “Dynamically reconfigurable complex emulsions via tunable interfacial tensions.” Nature 2015, 518, 520–524.
18. Koo, B.; Sletten, E.M.; Swager, T.M. “Efficient synthesis of functionalized poly(3-hexylthiophenes)s via lithium-bromine exchange.” Macromolecules 2015, 48, 229–235.
17. Sletten, E.M.; Swager, T.M. “Fluorofluorophores: fluorescent fluorous chemical tools spanning the visible spectrum.” J. Am. Chem. Soc. 2014, 136, 13574–13577.
Graduate Work
16. Tomlin, F.M.; Gordon, C.G.; Han, Y.; Wu, T.S.; Sletten, E.M.; Bertozzi, C.R. “Site specific incorporation of quadricyclane into a protein and photocleavage of the quadricyclane ligation adduct.” Bioorg. Med. Chem. Lett. 2018, 26, 5280–5290.
15. Sletten, E.M.; de Almeida, G.; Bertozzi, C.R. “A homologation approach to the synthesis of difluorinated cycloalkynes.” Org. Lett. 2014, 16, 1634–1637.
14. Agarwal, P.; van der Weijden, J.; Sletten, E.M.; Rabuka, D.; Bertozzi, C.R. “A Pictet-Spengler ligation for protein chemical modification.” Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 46–51.
13. Gordon, C.G.; Mackey, J.; Jewett, J.C.; Sletten, E.M.; Houk, K.N.; Bertozzi, C.R. “Reactivity of biarylazacyclooctynones in copper-free click chemistry.” J. Am. Chem. Soc. 2012, 134, 9199–9208.
12. Yao, J.Z.; Uttamapinant, C.; Poloukhtine, A.; Baskin, J.M.; Codelli, J.A.; Sletten, E.M.; Bertozzi, C.R.; Popik, V.V.; Ting, A,Y. “Fluorophore targeting to cellular proteins via enzyme-mediated azide ligation and strain-promoted cycloaddition.” J. Am. Chem. Soc. 2012, 134, 3720–3728.
11. de Almeida, G.; Sletten, E.M.; Nakamura, H.; Palaniappan, K.K.; Bertozzi, C.R. “Thiacycloalkynes for Cu-free click chemistry.” Angew. Chem. Int. Ed. 2012, 51, 2443–2447.
10. Sletten, E.M.; Bertozzi, C.R. “A bioorthogonal quadricyclane ligation.” J. Am. Chem. Soc. 2011, 133, 17570–17573.
9. Sletten, E.M.; Bertozzi, C.R. “From mechanism to mouse: a tale of two bioorthogonal reactions.” Acc. Chem. Res. 2011, 44, 666–676.
8. Sletten, E.M.; Nakamura, H.; Jewett, J.C.; Bertozzi, C.R. “Difluorobenzocyclooctyne: synthesis, characterization, and stabilization by beta-cyclodextrin.” J. Am. Chem. Soc. 2010, 132, 11799–11805.
7. Chang, P.V.; Dube, D.H.; Sletten, E.M.; Bertozzi, C.R. “A strategy for the selective imaging of glycans using caged metabolic precursors.” J. Am. Chem. Soc. 2010, 132, 9516–9518.
6. Jewett, J.C.; Sletten, E.M.; Bertozzi, C.R. “Rapid Cu-free click chemistry with readily synthesized biarylazacyclooctynones.” J. Am. Chem. Soc. 2010, 132, 3688–3690.
5. Chang, P.V.*; Prescher, J.A.*; Sletten, E.M.; Baskin, J.M.; Miller, I.A.; Agard, N.J.; Lo, A.; Bertozzi, C.R. “Copper-free click chemistry in living animals.” Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 1821–1826.
4. Sletten, E.M.; Bertozzi, C.R. “Bioorthogonal chemistry: fishing for selectivity in a sea of functionality.” Angew. Chem. Int. Ed. 2009, 48, 6974–6998.
3. Sletten, E.M.; Bertozzi, C.R. “A hydrophilic azacyclooctyne for Cu-free click chemistry.” Org. Lett. 2008, 10, 3097–3099.
Undergraduate Work
2. Kelly, C.B.; Colthart, A.M.; Constant, B.D.; Corning, S.R.; Dubois, L.N.; Genovese, J.T.; Radziewicz, J.L.; Sletten, E.M.; Whitaker, K.R.; Tilley, L.J. “Enabling the synthesis of perfluoroalkyl bicyclobutanes via 1,3 g-silyl elimination.” Org. Lett. 2011, 13, 1646–1649.
1. Sletten, E.M.; Liotta, L.J. “A flexible stereospecific synthesis of polyhydroxyated pyrrolizidines from commercially available pyranosides.” J. Org. Chem. 2006, 71, 1335–1343.
